当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Palladium-Catalyzed Asymmetric C–H Arylation for the Synthesis of Planar Chiral Benzothiophene-Fused Ferrocenes
ACS Catalysis ( IF 11.3 ) Pub Date : 2018-11-12 00:00:00 , DOI: 10.1021/acscatal.8b03912 Bing-Bin Xu 1 , Jie Ye 1 , Yu Yuan 1 , Wei-Liang Duan 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2018-11-12 00:00:00 , DOI: 10.1021/acscatal.8b03912 Bing-Bin Xu 1 , Jie Ye 1 , Yu Yuan 1 , Wei-Liang Duan 1
Affiliation
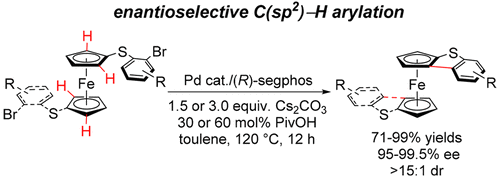
|
An intramolecular enantioselective C–H arylation of ferrocenyl aryl sulfides was performed with a palladium catalyst. Planar chiral thiophene compounds with a ferrocene skeleton were constructed in good enantioselectivities (up to 99% ee) and high yields, and the utility of the obtained chiral sulfides as ligands in catalytic reactions has been demonstrated.
中文翻译:

钯催化不对称CH杂化反应合成平面手性苯并噻吩基二茂铁
二茂铁基芳基硫醚的分子内对映选择性CH芳基化反应是用钯催化剂进行的。具有良好的对映选择性(高达99%ee)和高收率构建了具有二茂铁骨架的平面手性噻吩化合物,并且已经证明了所获得的手性硫化物作为催化反应中的配体的用途。
更新日期:2018-11-12
中文翻译:

钯催化不对称CH杂化反应合成平面手性苯并噻吩基二茂铁
二茂铁基芳基硫醚的分子内对映选择性CH芳基化反应是用钯催化剂进行的。具有良好的对映选择性(高达99%ee)和高收率构建了具有二茂铁骨架的平面手性噻吩化合物,并且已经证明了所获得的手性硫化物作为催化反应中的配体的用途。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号