当前位置:
X-MOL 学术
›
Asian J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
N‐Iodosuccinimide as Bifunctional Reagent in (E)‐Selective C(sp2)−H Sulfonylation of Styrenes
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2018-12-04 , DOI: 10.1002/ajoc.201800644 Milan Pramanik 1 , Khokan Choudhuri 1 , Prasenjit Mal 1
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2018-12-04 , DOI: 10.1002/ajoc.201800644 Milan Pramanik 1 , Khokan Choudhuri 1 , Prasenjit Mal 1
Affiliation
|
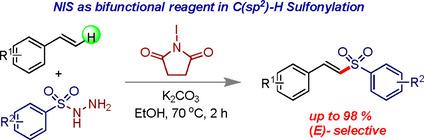
Herein we report the use of N‐iodosuccinimide (NIS) as a bifunctional reagent for a regio‐ and (E)‐selective C(sp2)‐H sulfonylation reaction of styrenes. Styrenes and sulfonyl hydrazides treated with NIS and potassium carbonate in ethanol at 70 °C resulted in (E)‐vinyl sulfones exclusively with good to excellent yields. NIS, plays a dual role to generate sulfonyl radical from sulfonyl hydrazides at an initial stage and finally gives β‐iodosulfone intermediate which was further converted to (E)‐vinyl sulfones. Overall, a sustainable method for mild, metal free, convenient, one pot and direct synthesis of (E)‐vinyl sulfones from styrenes are demonstrated via a C−S coupling reaction.
中文翻译:

N-碘代琥珀酰亚胺在苯乙烯的(E)-选择性C(sp2)-H磺酰化中作为双功能试剂
本文中,我们报道了使用N-碘琥珀酰亚胺(NIS)作为双功能试剂进行苯乙烯的区域和(E)选择性C(sp 2)-H磺酰化反应。在70°C下用NIS和碳酸钾在乙醇中处理过的苯乙烯和磺酰肼生成(E)-乙烯基砜,其收率非常好。NIS起着双重作用,在初始阶段从磺酰肼生成磺酰基,最后得到β-碘砜中间体,该中间体进一步转化为(E)-乙烯基砜。总体而言,一种温和,无金属,方便,一锅直接合成(E的可持续方法苯乙烯-乙烯基砜通过C-S偶联反应得到证明。
更新日期:2018-12-04
中文翻译:

N-碘代琥珀酰亚胺在苯乙烯的(E)-选择性C(sp2)-H磺酰化中作为双功能试剂
本文中,我们报道了使用N-碘琥珀酰亚胺(NIS)作为双功能试剂进行苯乙烯的区域和(E)选择性C(sp 2)-H磺酰化反应。在70°C下用NIS和碳酸钾在乙醇中处理过的苯乙烯和磺酰肼生成(E)-乙烯基砜,其收率非常好。NIS起着双重作用,在初始阶段从磺酰肼生成磺酰基,最后得到β-碘砜中间体,该中间体进一步转化为(E)-乙烯基砜。总体而言,一种温和,无金属,方便,一锅直接合成(E的可持续方法苯乙烯-乙烯基砜通过C-S偶联反应得到证明。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号