Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The entropic force generated by intrinsically disordered segments tunes protein function
Nature ( IF 50.5 ) Pub Date : 2018-11-01 , DOI: 10.1038/s41586-018-0699-5 Nicholas D. Keul , Krishnadev Oruganty , Elizabeth T. Schaper Bergman , Nathaniel R. Beattie , Weston E. McDonald , Renuka Kadirvelraj , Michael L. Gross , Robert S. Phillips , Stephen C. Harvey , Zachary A. Wood
Nature ( IF 50.5 ) Pub Date : 2018-11-01 , DOI: 10.1038/s41586-018-0699-5 Nicholas D. Keul , Krishnadev Oruganty , Elizabeth T. Schaper Bergman , Nathaniel R. Beattie , Weston E. McDonald , Renuka Kadirvelraj , Michael L. Gross , Robert S. Phillips , Stephen C. Harvey , Zachary A. Wood
|
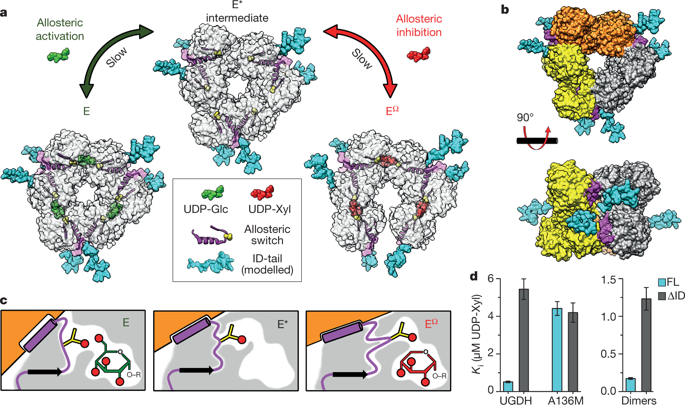
Protein structures are dynamic and can explore a large conformational landscape1,2. Only some of these structural substates are important for protein function (such as ligand binding, catalysis and regulation)3–5. How evolution shapes the structural ensemble to optimize a specific function is poorly understood3,4. One of the constraints on the evolution of proteins is the stability of the folded ‘native’ state. Despite this, 44% of the human proteome contains intrinsically disordered peptide segments greater than 30 residues in length6, the majority of which have no known function7–9. Here we show that the entropic force produced by an intrinsically disordered carboxy terminus (ID-tail) shifts the conformational ensemble of human UDP-α-d-glucose-6-dehydrogenase (UGDH) towards a substate with a high affinity for an allosteric inhibitor. The function of the ID-tail does not depend on its sequence or chemical composition. Instead, the affinity enhancement can be accurately predicted based on the length of the intrinsically disordered segment, and is consistent with the entropic force generated by an unstructured peptide attached to the protein surface10–13. Our data show that the unfolded state of the ID-tail rectifies the dynamics and structure of UGDH to favour inhibitor binding. Because this entropic rectifier does not have any sequence or structural constraints, it is an easily acquired adaptation. This model implies that evolution selects for disordered segments to tune the energy landscape of proteins, which may explain the persistence of intrinsic disorder in the proteome.The carboxy terminus of human UDP-α-d-glucose-6-dehydrogenase is structurally disordered, but has sequence-independent effects on the conformation of the enzyme and binding of an allosteric inhibitor, suggesting a reason for the persistence of intrinsically disordered peptide segments in the proteome.
中文翻译:

内在无序片段产生的熵力调节蛋白质功能
蛋白质结构是动态的,可以探索大的构象景观 1,2。只有其中一些结构亚状态对蛋白质功能(例如配体结合、催化和调节)很重要3-5。人们对进化如何塑造结构整体以优化特定功能知之甚少3,4。蛋白质进化的限制之一是折叠“天然”状态的稳定性。尽管如此,44% 的人类蛋白质组包含长度超过 30 个残基的内在无序肽段 6,其中大多数没有已知的功能 7-9。在这里,我们展示了由本质上无序的羧基末端(ID-tail)产生的熵力将人 UDP-α-d-glucose-6-dehydrogenase (UGDH) 的构象集合转变为对变构抑制剂具有高亲和力的亚态. ID-tail 的功能不依赖于它的序列或化学成分。相反,亲和力增强可以根据固有无序片段的长度准确预测,并且与附着在蛋白质表面的非结构化肽产生的熵力一致 10-13。我们的数据表明,ID 尾的展开状态纠正了 UGDH 的动力学和结构,有利于抑制剂结合。因为这个熵整流器没有任何序列或结构限制,所以很容易获得适应。该模型暗示进化选择无序片段来调整蛋白质的能量景观,这可以解释蛋白质组中内在无序的持续存在。人类 UDP-α-d-葡萄糖-6-脱氢酶的羧基末端在结构上是无序的,
更新日期:2018-11-01
中文翻译:

内在无序片段产生的熵力调节蛋白质功能
蛋白质结构是动态的,可以探索大的构象景观 1,2。只有其中一些结构亚状态对蛋白质功能(例如配体结合、催化和调节)很重要3-5。人们对进化如何塑造结构整体以优化特定功能知之甚少3,4。蛋白质进化的限制之一是折叠“天然”状态的稳定性。尽管如此,44% 的人类蛋白质组包含长度超过 30 个残基的内在无序肽段 6,其中大多数没有已知的功能 7-9。在这里,我们展示了由本质上无序的羧基末端(ID-tail)产生的熵力将人 UDP-α-d-glucose-6-dehydrogenase (UGDH) 的构象集合转变为对变构抑制剂具有高亲和力的亚态. ID-tail 的功能不依赖于它的序列或化学成分。相反,亲和力增强可以根据固有无序片段的长度准确预测,并且与附着在蛋白质表面的非结构化肽产生的熵力一致 10-13。我们的数据表明,ID 尾的展开状态纠正了 UGDH 的动力学和结构,有利于抑制剂结合。因为这个熵整流器没有任何序列或结构限制,所以很容易获得适应。该模型暗示进化选择无序片段来调整蛋白质的能量景观,这可以解释蛋白质组中内在无序的持续存在。人类 UDP-α-d-葡萄糖-6-脱氢酶的羧基末端在结构上是无序的,






























 京公网安备 11010802027423号
京公网安备 11010802027423号