Journal of Colloid and Interface Science ( IF 9.4 ) Pub Date : 2018-11-08 , DOI: 10.1016/j.jcis.2018.11.021 Haris Khan , John M Seddon , Robert V Law , Nicholas J Brooks , Eric Robles , João T Cabral , Oscar Ces
|
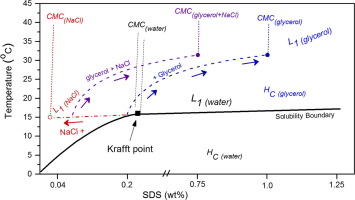
The effect of glycerol with sodium chloride (NaCl) on the phase behaviour of sodium dodecyl sulfate (SDS) near the Krafft point was studied by surface tension analysis using the pendant drop method. The critical micelle concentration (CMC) and Krafft Temperature (TK) of SDS in water: glycerol mixtures, across the full composition range, and in NaCl solutions within 0.005–0.1 M were obtained. The pendant drop method successfully allowed us to determine the Krafft point of SDS in high glycerol systems where other traditional methods (e.g. conductivity) have been ineffective. Overall the addition of glycerol increases the CMC and the TK, thus shifting the Krafft point of SDS to higher temperatures (increasing crystallisation temperatures) and higher SDS content in the presence of glycerol, which is interpreted as a result of the reduction in solvent polarity which opposes micellization. The addition of NaCl to the SDS – water-glycerol systems brings the CMC back down, while having no significant effect on the TK. Our results establish a robust route for tuning the Krafft point of model surfactant SDS by adjusting solvent quality and salt content.
中文翻译:

表面张力作用下氯化钠甘油对十二烷基硫酸钠克拉夫点的影响
采用悬滴法通过表面张力分析研究了甘油与氯化钠(NaCl)对十二烷基硫酸钠(SDS)在Krafft点附近的相行为的影响。在整个组成范围内以及在NaCl溶液中,获得的SDS在水:甘油混合物中的临界胶束浓度(CMC)和Krafft温度(T K)在0.005-0.1 M之间。悬滴法成功地使我们能够确定在其他传统方法(例如电导率)无效的高甘油系统中,SDS的Krafft点。总体而言,添加甘油会增加CMC和T K因此,在存在甘油的情况下,将SDS的Krafft点转移到更高的温度(增加结晶温度)和更高的SDS含量,这被解释为与胶束化相反的溶剂极性降低的结果。在SDS-水-甘油系统中添加NaCl可使CMC下降,而对T K却无明显影响。我们的结果为通过调节溶剂质量和盐含量来调节模型表面活性剂SDS的Krafft点建立了可靠的途径。






























 京公网安备 11010802027423号
京公网安备 11010802027423号