Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Complement C3aR Inactivation Attenuates Tau Pathology and Reverses an Immune Network Deregulated in Tauopathy Models and Alzheimer's Disease.
Neuron ( IF 14.7 ) Pub Date : 2018-11-08 , DOI: 10.1016/j.neuron.2018.10.031 Alexandra Litvinchuk 1 , Ying-Wooi Wan 2 , Dan B Swartzlander 3 , Fading Chen 3 , Allysa Cole 3 , Nicholas E Propson 4 , Qian Wang 5 , Bin Zhang 5 , Zhandong Liu 6 , Hui Zheng 7
Neuron ( IF 14.7 ) Pub Date : 2018-11-08 , DOI: 10.1016/j.neuron.2018.10.031 Alexandra Litvinchuk 1 , Ying-Wooi Wan 2 , Dan B Swartzlander 3 , Fading Chen 3 , Allysa Cole 3 , Nicholas E Propson 4 , Qian Wang 5 , Bin Zhang 5 , Zhandong Liu 6 , Hui Zheng 7
Affiliation
|
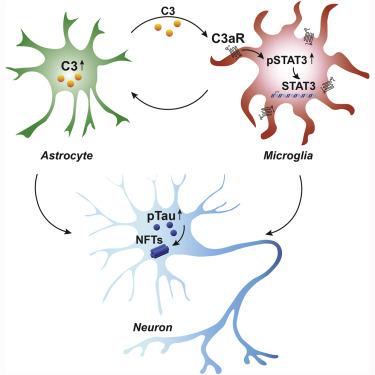
Strong evidence implicates the complement pathway as an important contributor to amyloid pathology in Alzheimer's disease (AD); however, the role of complement in tau modulation remains unclear. Here we show that the expression of C3 and C3a receptor (C3aR1) are positively correlated with cognitive decline and Braak staging in human AD brains. Deletion of C3ar1 in PS19 mice results in the rescue of tau pathology and attenuation of neuroinflammation, synaptic deficits, and neurodegeneration. Through RNA sequencing and cell-type-specific transcriptomic analysis, we identify a C3aR-dependent transcription factor network that regulates a reactive glial switch whose inactivation ameliorates disease-associated microglia and neurotoxic astrocyte signatures. Strikingly, this C3aR network includes multiple genes linked to late-onset AD. Mechanistically, we identify STAT3 as a direct target of C3-C3aR signaling that functionally mediates tau pathogenesis. All together our findings demonstrate a crucial role for activation of the C3-C3aR network in mediating neuroinflammation and tau pathology.
中文翻译:

补体C3aR失活减弱了Tau病理,并逆转了在Tauopathy模型和阿尔茨海默氏病模型中失调的免疫网络。
有力的证据表明补体途径是阿尔茨海默氏病(AD)中淀粉样蛋白病理学的重要贡献者;然而,补体在tau调制中的作用尚不清楚。在这里,我们显示C3和C3a受体(C3aR1)的表达与人类AD大脑中的认知能力下降和Braak分期呈正相关。PS19小鼠中C3ar1的缺失可导致tau病理的挽救,并减轻神经炎症,突触缺陷和神经退行性变。通过RNA测序和特定于细胞类型的转录组学分析,我们确定了一个C3aR依赖的转录因子网络,该网络调节反应性神经胶质开关,其失活可改善与疾病相关的小胶质细胞和神经毒性星形胶质细胞信号。令人惊讶的是,这个C3aR网络包含与晚期AD相关的多个基因。机械上,我们确定STAT3作为C3-C3aR信号传导的直接靶标,在功能上介导tau发病机理。所有我们的发现共同证明了在介导神经炎症和tau病理过程中激活C3-C3aR网络的关键作用。
更新日期:2018-11-09
中文翻译:

补体C3aR失活减弱了Tau病理,并逆转了在Tauopathy模型和阿尔茨海默氏病模型中失调的免疫网络。
有力的证据表明补体途径是阿尔茨海默氏病(AD)中淀粉样蛋白病理学的重要贡献者;然而,补体在tau调制中的作用尚不清楚。在这里,我们显示C3和C3a受体(C3aR1)的表达与人类AD大脑中的认知能力下降和Braak分期呈正相关。PS19小鼠中C3ar1的缺失可导致tau病理的挽救,并减轻神经炎症,突触缺陷和神经退行性变。通过RNA测序和特定于细胞类型的转录组学分析,我们确定了一个C3aR依赖的转录因子网络,该网络调节反应性神经胶质开关,其失活可改善与疾病相关的小胶质细胞和神经毒性星形胶质细胞信号。令人惊讶的是,这个C3aR网络包含与晚期AD相关的多个基因。机械上,我们确定STAT3作为C3-C3aR信号传导的直接靶标,在功能上介导tau发病机理。所有我们的发现共同证明了在介导神经炎症和tau病理过程中激活C3-C3aR网络的关键作用。































 京公网安备 11010802027423号
京公网安备 11010802027423号