当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Inter/Intramolecular Bonds in TH5+ (T = C/Si/Ge): H2 as Tetrel Bond Acceptor and the Uniqueness of Carbon Bonds
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2018-11-08 00:00:00 , DOI: 10.1021/acs.jpca.8b09778 Sharon Priya Gnanasekar 1 , Elangannan Arunan 1
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2018-11-08 00:00:00 , DOI: 10.1021/acs.jpca.8b09778 Sharon Priya Gnanasekar 1 , Elangannan Arunan 1
Affiliation
|
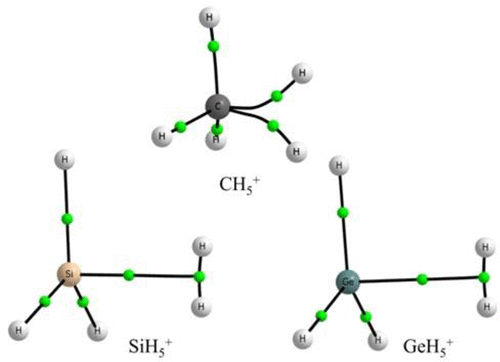
Atoms in molecules (AIM), natural bond orbital (NBO), and normal coordinate analysis have been carried out at the global minimum structures of TH5+ (T = C/Si/Ge). All these analyses lead to a consistent structure for these three protonated TH4 molecules. The CH5+ has a structure with three short and two long C–H covalent bonds and no H–H bond. Hence, the popular characterization of protonated methane as a weakly bound CH3+ and H2 is inconsistent with these results. However, SiH5+ and GeH5+ are both indeed a complex formed between TH3+ and H2 stabilized by a tetrel bond, with the H2 being the tetrel bond acceptor. The three-center-two-electron bond (3c–2e) in CH5+ has an open structure, which can be characterized as a V-type 3c–2e bond and that found in SiH5+ and GeH5+ is a T-type 3c–2e bond. This difference could be understood based on the typical C–H, Si–H, Ge–H, and H–H bond and the tetrel bond energies. This analysis explains the trend observed in proton affinity of TH4 which appears counterintuitive, GeH4 > SiH4 > CH4. Carbon is selective in forming a “tetrel bond” and when it does, it might be worthwhile to highlight it as a “carbon bond”.
中文翻译:

TH 5 +(T = C / Si / Ge):H 2作为Tetrel键受体的分子间/分子内键和碳键的唯一性
已经在TH 5 +(T = C / Si / Ge)的整体最小结构下进行了分子原子(AIM),自然键轨道(NBO)和法向坐标分析。所有这些分析导致这三个质子化的TH 4分子具有一致的结构。CH 5 +具有三个短和两个长的C–H共价键而没有H–H键的结构。因此,质子化甲烷作为弱键合的CH 3 +和H 2的普遍表征与这些结果不一致。但是,SiH 5 +和GeH 5 +确实是TH 3 +和H 2之间形成的络合物通过一个Tetrel键稳定,H 2是Tetrel键受体。CH 5 +中的三中心二电子键(3c-2e)具有开放结构,可以表征为V型3c-2e键,SiH 5 +和GeH 5 +中的T为T -3c-2e型键。可以基于典型的C–H,Si–H,Ge–H和H–H键以及the铁键的能量来理解这种差异。该分析解释了在TH 4的质子亲和力中观察到的趋势,该趋势似乎与直觉相反, GeH 4 > SiH 4 > CH 4。碳在形成“ tetrel键”时具有选择性,当它形成时,可能有必要将其突出显示为“碳键”。
更新日期:2018-11-08
中文翻译:

TH 5 +(T = C / Si / Ge):H 2作为Tetrel键受体的分子间/分子内键和碳键的唯一性
已经在TH 5 +(T = C / Si / Ge)的整体最小结构下进行了分子原子(AIM),自然键轨道(NBO)和法向坐标分析。所有这些分析导致这三个质子化的TH 4分子具有一致的结构。CH 5 +具有三个短和两个长的C–H共价键而没有H–H键的结构。因此,质子化甲烷作为弱键合的CH 3 +和H 2的普遍表征与这些结果不一致。但是,SiH 5 +和GeH 5 +确实是TH 3 +和H 2之间形成的络合物通过一个Tetrel键稳定,H 2是Tetrel键受体。CH 5 +中的三中心二电子键(3c-2e)具有开放结构,可以表征为V型3c-2e键,SiH 5 +和GeH 5 +中的T为T -3c-2e型键。可以基于典型的C–H,Si–H,Ge–H和H–H键以及the铁键的能量来理解这种差异。该分析解释了在TH 4的质子亲和力中观察到的趋势,该趋势似乎与直觉相反, GeH 4 > SiH 4 > CH 4。碳在形成“ tetrel键”时具有选择性,当它形成时,可能有必要将其突出显示为“碳键”。

































 京公网安备 11010802027423号
京公网安备 11010802027423号