当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Rossmann-Fold Methyltransferases: Taking a “β-Turn” around Their Cofactor, S-Adenosylmethionine
Biochemistry ( IF 2.9 ) Pub Date : 2018-11-08 00:00:00 , DOI: 10.1021/acs.biochem.8b00994 Bhanu Pratap Singh Chouhan 1 , Shayida Maimaiti 1 , Madhuri Gade 1 , Paola Laurino 1
Biochemistry ( IF 2.9 ) Pub Date : 2018-11-08 00:00:00 , DOI: 10.1021/acs.biochem.8b00994 Bhanu Pratap Singh Chouhan 1 , Shayida Maimaiti 1 , Madhuri Gade 1 , Paola Laurino 1
Affiliation
|
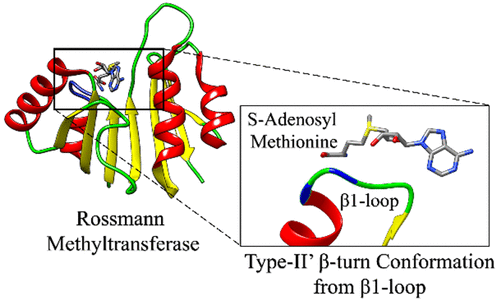
Methyltransferases (MTases) are superfamilies of enzymes that catalyze the transfer of a methyl group from S-adenosylmethionine (SAM), a nucleoside-based cofactor, to a wide variety of substrates such as DNA, RNA, proteins, small molecules, and lipids. Depending upon their structural features, the MTases can be further classified into different classes; we consider exclusively the largest class of MTases, the Rossmann-fold MTases. It has been shown that the nucleoside cofactor-binding Rossmann enzymes, particularly the nicotinamide adenine dinucleotide (NAD)-, flavin adenine dinucleotide (FAD)-, and SAM-binding MTases enzymes, share common binding motifs that include a Gly-rich loop region that interacts with the cofactor and a highly conserved acidic residue (Asp/Glu) that interacts with the ribose moiety of the cofactor. Here, we observe that the Gly-rich loop region of the Rossmann MTases adapts a specific type II′ β-turn in the proximity of the cofactor (<4 Å), and it appears to be a key feature of these superfamilies. Additionally, we demonstrate that the conservation of this β-turn could play a critical role in the enzyme–cofactor interaction, thereby shedding new light on the structural conformation of the Gly-rich loop region from Rossmann MTases.
中文翻译:

Rossmann-fold甲基转移酶:围绕其辅因子S-腺苷甲硫氨酸进行“β-转”
甲基转移酶(MTase)是催化从S转移甲基的酶的超家族-腺苷甲硫氨酸(SAM),一种基于核苷的辅因子,可用于多种底物,例如DNA,RNA,蛋白质,小分子和脂质。根据它们的结构特征,MTase可以进一步分为不同的类别。我们仅考虑最大类的MTase,即罗斯曼倍数MTase。已经显示,结合核苷辅因子的Rossmann酶,特别是烟酰胺腺嘌呤二核苷酸(NAD),黄素腺嘌呤二核苷酸(FAD)和SAM结合MTase酶,具有共同的结合基序,包括富含Gly的环区。与辅因子相互作用的氨基酸残基与与辅因子的核糖部分相互作用的高度保守的酸性残基(Asp / Glu)相互作用。这里,我们观察到,罗斯曼MTases的富含Gly的环区域在辅因子(<4Å)附近适应了特定的II'型β-转角,这似乎是这些超家族的关键特征。此外,我们证明了这种β-转角的保守性可能在酶-辅因子相互作用中起关键作用,从而为来自Rossmann MTases的富含Gly的环区域的结构构象提供了新的思路。
更新日期:2018-11-08
中文翻译:

Rossmann-fold甲基转移酶:围绕其辅因子S-腺苷甲硫氨酸进行“β-转”
甲基转移酶(MTase)是催化从S转移甲基的酶的超家族-腺苷甲硫氨酸(SAM),一种基于核苷的辅因子,可用于多种底物,例如DNA,RNA,蛋白质,小分子和脂质。根据它们的结构特征,MTase可以进一步分为不同的类别。我们仅考虑最大类的MTase,即罗斯曼倍数MTase。已经显示,结合核苷辅因子的Rossmann酶,特别是烟酰胺腺嘌呤二核苷酸(NAD),黄素腺嘌呤二核苷酸(FAD)和SAM结合MTase酶,具有共同的结合基序,包括富含Gly的环区。与辅因子相互作用的氨基酸残基与与辅因子的核糖部分相互作用的高度保守的酸性残基(Asp / Glu)相互作用。这里,我们观察到,罗斯曼MTases的富含Gly的环区域在辅因子(<4Å)附近适应了特定的II'型β-转角,这似乎是这些超家族的关键特征。此外,我们证明了这种β-转角的保守性可能在酶-辅因子相互作用中起关键作用,从而为来自Rossmann MTases的富含Gly的环区域的结构构象提供了新的思路。










































 京公网安备 11010802027423号
京公网安备 11010802027423号