Journal of Catalysis ( IF 6.5 ) Pub Date : 2018-11-07 , DOI: 10.1016/j.jcat.2018.10.001
Xiao Jiang , Xiaowa Nie , Xiaoxing Wang , Haozhi Wang , Naoto Koizumi , Yonggang Chen , Xinwen Guo , Chunshan Song
|
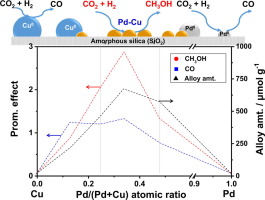
A strong synergetic effect was observed in our previous work on Pd-Cu bimetallic catalysts for CH3OH formation from CO2 hydrogenation when the Pd/(Pd + Cu) atomic ratio lied within 0.25–0.34. In the present study, the importance of Pd-Cu alloy in selective CH3OH promotion was evidenced and correlated with alloy contents quantitatively through X-ray diffraction (XRD), scanning transmission electron spectroscopy with energy-dispersive X-ray spectroscopy (STEM/EDS), and H2-O2 titration and N2O titration. The surface chemical properties of Pd-Cu combinations were characterized by H2-/CO2-temperature-programmed desorption (TPD), diffuse reflectance infrared FT spectroscopy (DRIFTS), and density functional theory (DFT), and experimentally evaluated along with monometallic counterparts. Detailed characterization results reveal a unique shift in adsorption towards weakly-bonded H2 and CO2 on Pd-Cu bimetallic surface which appear to correlate to the CH3OH promotion. DFT calculations on adsorption properties of H2 and CO2 show good agreement with the observation from TPD experiments. DFT study also provides insights into the impact of Pd-Cu combination on the activation and initial hydrogenation of CO2 to formate (HCOO∗) and hydrocarboxyl (COOH∗) intermediates. HCOO∗ formation was found to be kinetically more favored than COOH∗ on monometallic Cu and Pd-Cu surfaces. The lowest barrier for HCOO∗ formation was observed at Pd/(Pd + Cu) atomic ratio of 0.33, around which a good CO2 conversion and high methanol selectivity were achieved experimentally.
中文翻译:

Pd-Cu双金属效应协同促进CO 2加氢形成甲醇的起源
当我们以前的工作中,当Pd /(Pd + Cu)原子比在0.25-0.34之内时,对于由CO 2加氢形成CH 3 OH的Pd-Cu双金属催化剂,观察到了强大的协同作用。在本研究中,在选择性CH Pd-Cu合金的重要性3 OH促进被证实,并用合金含量定量通过X射线衍射(XRD)相关,扫描透射电子显微镜能量分散X射线光谱法(STEM / EDS),H 2 -O 2滴定和N 2 O滴定。通过H 2- / CO 2表征了Pd-Cu组合的表面化学性质。程序升温脱附(TPD),漫反射红外FT光谱(DRIFTS)和密度泛函理论(DFT),并与单金属对应物一起进行了实验评估。详细的表征结果显示,Pd-Cu双金属表面上的吸附向弱键合的H 2和CO 2的转移发生了独特变化,这似乎与CH 3 OH的促进作用相关。DFT对H 2和CO 2吸附性能的计算与TPD实验的观察结果吻合良好。DFT研究还提供了有关Pd-Cu组合对CO 2活化和初始加氢成甲酸(HCOO ∗)和氢羧基(COOH)的影响的见解。∗)中间体。发现在单金属Cu和Pd-Cu表面上HCOO *的形成在动力学上比COOH *更受青睐。在Pd /(Pd + Cu)原子比为0.33时,观察到最低的HCOO *形成势垒,通过实验可以实现良好的CO 2转化率和高甲醇选择性。































 京公网安备 11010802027423号
京公网安备 11010802027423号