当前位置:
X-MOL 学术
›
Nat. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Molecular structure of promoter-bound yeast TFIID.
Nature Communications ( IF 14.7 ) Pub Date : 2018-11-07 , DOI: 10.1038/s41467-018-07096-y Olga Kolesnikova , Adam Ben-Shem , Jie Luo , Jeff Ranish , Patrick Schultz , Gabor Papai
Nature Communications ( IF 14.7 ) Pub Date : 2018-11-07 , DOI: 10.1038/s41467-018-07096-y Olga Kolesnikova , Adam Ben-Shem , Jie Luo , Jeff Ranish , Patrick Schultz , Gabor Papai
|
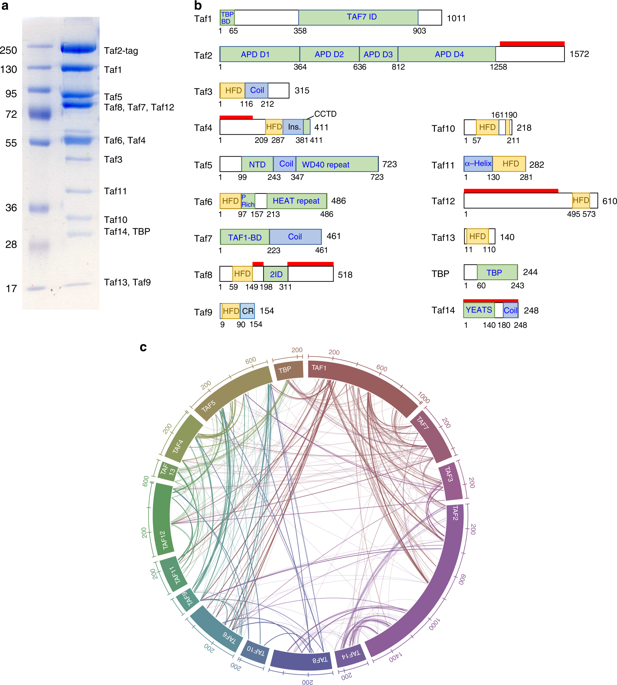
Transcription preinitiation complex assembly on the promoters of protein encoding genes is nucleated in vivo by TFIID composed of the TATA-box Binding Protein (TBP) and 13 TBP-associate factors (Tafs) providing regulatory and chromatin binding functions. Here we present the cryo-electron microscopy structure of promoter-bound yeast TFIID at a resolution better than 5 Å, except for a flexible domain. We position the crystal structures of several subunits and, in combination with cross-linking studies, describe the quaternary organization of TFIID. The compact tri lobed architecture is stabilized by a topologically closed Taf5-Taf6 tetramer. We confirm the unique subunit stoichiometry prevailing in TFIID and uncover a hexameric arrangement of Tafs containing a histone fold domain in the Twin lobe.
中文翻译:

启动子结合的酵母TFIID的分子结构。
TFIID在体内编码蛋白质编码基因的启动子上的转录预起始复合物,该TFIID由TATA盒结合蛋白(TBP)和13种TBP相关因子(Tafs)组成,提供调节和染色质结合功能。在这里,我们提出了与启动子结合的酵母TFIID的低温电子显微镜结构,其分辨率优于5Å,除了柔性域。我们定位几个亚基的晶体结构,并与交联研究相结合,描述了TFIID的四级组织。紧凑的三叶架构通过拓扑封闭的Taf5-Taf6四聚体得以稳定。我们证实了在TFIID中普遍存在的独特的亚基化学计量,并揭示了在双叶中含有组蛋白折叠域的Tafs的六聚体排列。
更新日期:2018-11-07
中文翻译:

启动子结合的酵母TFIID的分子结构。
TFIID在体内编码蛋白质编码基因的启动子上的转录预起始复合物,该TFIID由TATA盒结合蛋白(TBP)和13种TBP相关因子(Tafs)组成,提供调节和染色质结合功能。在这里,我们提出了与启动子结合的酵母TFIID的低温电子显微镜结构,其分辨率优于5Å,除了柔性域。我们定位几个亚基的晶体结构,并与交联研究相结合,描述了TFIID的四级组织。紧凑的三叶架构通过拓扑封闭的Taf5-Taf6四聚体得以稳定。我们证实了在TFIID中普遍存在的独特的亚基化学计量,并揭示了在双叶中含有组蛋白折叠域的Tafs的六聚体排列。













































 京公网安备 11010802027423号
京公网安备 11010802027423号