当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
An Enzyme Cascade for Selective Modification of Tyrosine Residues in Structurally Diverse Peptides and Proteins
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2016-02-25 , DOI: 10.1021/jacs.5b10928 Anna-Winona Struck 1 , Matthew R Bennett 1 , Sarah A Shepherd 1 , Brian J C Law 1 , Ying Zhuo 1 , Lu Shin Wong 1 , Jason Micklefield 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2016-02-25 , DOI: 10.1021/jacs.5b10928 Anna-Winona Struck 1 , Matthew R Bennett 1 , Sarah A Shepherd 1 , Brian J C Law 1 , Ying Zhuo 1 , Lu Shin Wong 1 , Jason Micklefield 1
Affiliation
|
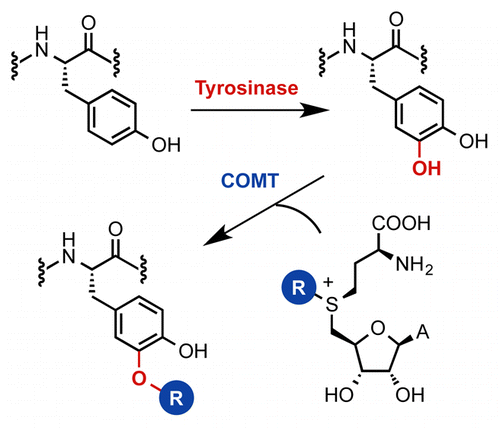
Bioorthogonal chemistry enables a specific moiety in a complex biomolecule to be selectively modified in the presence of many reactive functional groups and other cellular entities. Such selectivity has become indispensable in biology, enabling biomolecules to be derivatized, conjugated, labeled, or immobilized for imaging, biochemical assays, or therapeutic applications. Methyltransferase enzymes (MTase) that accept analogues of the cofactor S-adenosyl methionine have been widely deployed for alkyl-diversification and bioorthogonal labeling. However, MTases typically possess tight substrate specificity. Here we introduce a more flexible methodology for selective derivatization of phenolic moieties in complex biomolecules. Our approach relies on the tandem enzymatic reaction of a fungal tyrosinase and the mammalian catechol-O-methyltransferase (COMT), which can effect the sequential hydroxylation of the phenolic group to give an intermediate catechol moiety that is subsequently O-alkylated. When used in this combination, the alkoxylation is highly selective for tyrosine residues in peptides and proteins, yet remarkably tolerant to changes in the peptide sequence. Tyrosinase-COMT are shown to provide highly versatile and regioselective modification of a diverse range of substrates including peptide antitumor agents, hormones, cyclic peptide antibiotics, and model proteins.
中文翻译:

选择性修饰结构多样肽和蛋白质中酪氨酸残基的酶级联反应
生物正交化学能够在存在许多反应性官能团和其他细胞实体的情况下选择性地修饰复杂生物分子中的特定部分。这种选择性在生物学中已变得不可或缺,使生物分子能够被衍生、缀合、标记或固定用于成像、生化分析或治疗应用。接受辅因子 S-腺苷甲硫氨酸类似物的甲基转移酶 (MTase) 已广泛用于烷基多样化和生物正交标记。然而,MTases 通常具有严格的底物特异性。在这里,我们介绍了一种更灵活的方法,用于选择性衍生复杂生物分子中的酚类部分。我们的方法依赖于真菌酪氨酸酶和哺乳动物儿茶酚-O-甲基转移酶 (COMT) 的串联酶促反应,这可以影响酚基的顺序羟基化,从而产生随后被 O-烷基化的中间儿茶酚部分。当用于这种组合时,烷氧基化对肽和蛋白质中的酪氨酸残基具有高度选择性,但对肽序列的变化具有显着的耐受性。酪氨酸酶-COMT 被证明可以对多种底物提供高度通用和区域选择性的修饰,包括肽抗肿瘤剂、激素、环肽抗生素和模型蛋白。烷氧基化对肽和蛋白质中的酪氨酸残基具有高度选择性,但对肽序列的变化具有显着的耐受性。酪氨酸酶-COMT 被证明可以对多种底物提供高度通用和区域选择性的修饰,包括肽抗肿瘤剂、激素、环肽抗生素和模型蛋白。烷氧基化对肽和蛋白质中的酪氨酸残基具有高度选择性,但对肽序列的变化具有显着的耐受性。酪氨酸酶-COMT 被证明可以对多种底物提供高度通用和区域选择性的修饰,包括肽抗肿瘤剂、激素、环肽抗生素和模型蛋白。
更新日期:2016-02-25
中文翻译:

选择性修饰结构多样肽和蛋白质中酪氨酸残基的酶级联反应
生物正交化学能够在存在许多反应性官能团和其他细胞实体的情况下选择性地修饰复杂生物分子中的特定部分。这种选择性在生物学中已变得不可或缺,使生物分子能够被衍生、缀合、标记或固定用于成像、生化分析或治疗应用。接受辅因子 S-腺苷甲硫氨酸类似物的甲基转移酶 (MTase) 已广泛用于烷基多样化和生物正交标记。然而,MTases 通常具有严格的底物特异性。在这里,我们介绍了一种更灵活的方法,用于选择性衍生复杂生物分子中的酚类部分。我们的方法依赖于真菌酪氨酸酶和哺乳动物儿茶酚-O-甲基转移酶 (COMT) 的串联酶促反应,这可以影响酚基的顺序羟基化,从而产生随后被 O-烷基化的中间儿茶酚部分。当用于这种组合时,烷氧基化对肽和蛋白质中的酪氨酸残基具有高度选择性,但对肽序列的变化具有显着的耐受性。酪氨酸酶-COMT 被证明可以对多种底物提供高度通用和区域选择性的修饰,包括肽抗肿瘤剂、激素、环肽抗生素和模型蛋白。烷氧基化对肽和蛋白质中的酪氨酸残基具有高度选择性,但对肽序列的变化具有显着的耐受性。酪氨酸酶-COMT 被证明可以对多种底物提供高度通用和区域选择性的修饰,包括肽抗肿瘤剂、激素、环肽抗生素和模型蛋白。烷氧基化对肽和蛋白质中的酪氨酸残基具有高度选择性,但对肽序列的变化具有显着的耐受性。酪氨酸酶-COMT 被证明可以对多种底物提供高度通用和区域选择性的修饰,包括肽抗肿瘤剂、激素、环肽抗生素和模型蛋白。































 京公网安备 11010802027423号
京公网安备 11010802027423号