当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantioselective Synthesis of a Cyclopropane Derivative of Spliceostatin A and Evaluation of Bioactivity
Organic Letters ( IF 4.9 ) Pub Date : 2018-11-05 00:00:00 , DOI: 10.1021/acs.orglett.8b03228 Arun K Ghosh 1 , Guddeti Chandrashekar Reddy 1 , Satish Kovela 1 , Nicola Relitti 1 , Veronica K Urabe 2 , Beth E Prichard 2 , Melissa S Jurica 2
Organic Letters ( IF 4.9 ) Pub Date : 2018-11-05 00:00:00 , DOI: 10.1021/acs.orglett.8b03228 Arun K Ghosh 1 , Guddeti Chandrashekar Reddy 1 , Satish Kovela 1 , Nicola Relitti 1 , Veronica K Urabe 2 , Beth E Prichard 2 , Melissa S Jurica 2
Affiliation
|
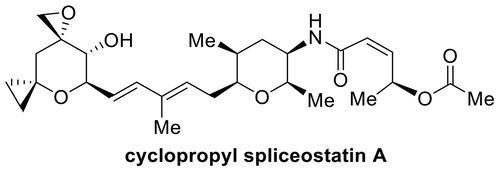
Spliceostatin A is a potent inhibitor of spliceosomes and exhibits excellent anticancer activity against multiple human cancer cell lines. We describe here the design and synthesis of a stable cyclopropane derivative of spliceostatin A. The synthesis involved a cross-metathesis or a Suzuki cross-coupling reaction as the key step. The functionalized epoxy alcohol ring was constructed from commercially available optically active tri-O-acetyl-d-glucal. The biological properties of the cyclopropyl derivative revealed that it is active in human cells and inhibits splicing in vitro comparable to spliceostatin A.
中文翻译:

剪接抑素A环丙烷衍生物的对映选择性合成及生物活性评价
Spliceostatin A 是一种有效的剪接体抑制剂,对多种人类癌细胞系表现出优异的抗癌活性。我们在此描述了剪接抑素A的稳定环丙烷衍生物的设计和合成。该合成涉及交叉复分解或Suzuki交叉偶联反应作为关键步骤。官能化环氧醇环由市售的光学活性三-O-乙酰基-d-葡萄糖构建。环丙基衍生物的生物学特性表明,它在人体细胞中具有活性,并且在体外抑制剪接的作用与剪接抑素 A 相当。
更新日期:2018-11-05
中文翻译:

剪接抑素A环丙烷衍生物的对映选择性合成及生物活性评价
Spliceostatin A 是一种有效的剪接体抑制剂,对多种人类癌细胞系表现出优异的抗癌活性。我们在此描述了剪接抑素A的稳定环丙烷衍生物的设计和合成。该合成涉及交叉复分解或Suzuki交叉偶联反应作为关键步骤。官能化环氧醇环由市售的光学活性三-O-乙酰基-d-葡萄糖构建。环丙基衍生物的生物学特性表明,它在人体细胞中具有活性,并且在体外抑制剪接的作用与剪接抑素 A 相当。































 京公网安备 11010802027423号
京公网安备 11010802027423号