当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis and Characterization of cyclo-Pentazolate Salts of NH4+, NH3OH+, N2H5+, C(NH2)3+, and N(CH3)4+
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-11-05 , DOI: 10.1021/jacs.8b05106 Chen Yang 1 , Chong Zhang 1 , Zhansheng Zheng 1 , Chao Jiang 1 , Jun Luo 1 , Yang Du 1 , Bingcheng Hu 1 , Chengguo Sun 1, 2 , Karl O. Christe 1, 3
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-11-05 , DOI: 10.1021/jacs.8b05106 Chen Yang 1 , Chong Zhang 1 , Zhansheng Zheng 1 , Chao Jiang 1 , Jun Luo 1 , Yang Du 1 , Bingcheng Hu 1 , Chengguo Sun 1, 2 , Karl O. Christe 1, 3
Affiliation
|
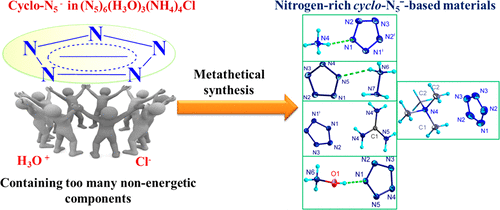
A breakthrough in polynitrogen chemistry was recently achieved by our bulk synthesis of (N5)6(H3O)3(NH4)4Cl in which the cyclo-pentazolate anions were stabilized extensively by hydrogen bridges with the NH4+ and OH3+ cations. Significant efforts have been carried out to replace these nonenergetic cations and the Cl- anion by more energetic cations. In this paper, the metathetical syntheses of cyclo-pentazolate salts containing the simple nitrogen-rich cations NH4+, NH3OH+, N2H5+, C(NH2)3+, and N(CH3)4+ are reported. These salts were characterized by their crystal structures; vibrational, mass, and multinuclear NMR spectra; thermal stability measurements; sensitivity data; and performance calculations. It is shown that the cyclo-pentazolates are more energetic than the corresponding azides but are thermally less stable decomposing in the range of 80 °C to 105 °C. As explosives, the hydrazinium and hydroxyl ammonium salts are predicted to match the detonation pressure of RDX but exhibit significantly higher detonation velocities than RDX and HMX with comparable impact and friction sensitivities. Although the ammonium salt has a lower detonation pressure than RDX, its detonation velocity also exceeds those of RDX and HMX. As a rocket propellant, the hydrazinium and hydroxyl ammonium salts are predicted to exceed the performances of RDX and HMX. The crystal structures show that the cyclo-pentazolate anions are generally stabilized by hydrogen bonds to the cations, except for the N(CH3)4+ salt which also exhibits strong cation-π interactions. This difference in the anion stabilization is also detectable in the vibrational spectra which show for the N(CH3)4+ salt a decrease in the cyclo-N5- stretching vibrations of about 20 cm-1.
中文翻译:

NH4+、NH3OH+、N2H5+、C(NH2)3+ 和 N(CH3)4+ 的环戊唑酸盐的合成和表征
我们最近通过大量合成 (N5)6(H3O)3(NH4)4Cl 实现了多氮化学的突破,其中环五唑阴离子通过与 NH4+ 和 OH3+ 阳离子的氢桥广泛稳定。已经进行了大量努力以用能量更高的阳离子代替这些非能量阳离子和氯阴离子。本文报道了含有简单富氮阳离子 NH4+、NH3OH+、N2H5+、C(NH2)3+ 和 N(CH3)4+ 的环戊唑盐的复分解合成。这些盐的特征在于它们的晶体结构;振动、质量和多核 NMR 光谱;热稳定性测量;敏感性数据;和性能计算。结果表明,环戊唑酯比相应的叠氮化物更具能量,但在 80°C 至 105°C 的范围内分解时热稳定性较差。作为炸药,肼盐和羟铵盐预计与 RDX 的爆轰压力相匹配,但其爆速明显高于 RDX 和 HMX,具有相当的冲击和摩擦敏感性。虽然铵盐的爆轰压力比RDX低,但其爆速也超过了RDX和HMX。作为火箭推进剂,预计肼盐和羟铵盐的性能将超过 RDX 和 HMX。晶体结构表明,除了 N(CH3)4+ 盐也表现出强阳离子-π 相互作用外,环戊唑根阴离子通常通过与阳离子的氢键稳定。
更新日期:2018-11-05
中文翻译:

NH4+、NH3OH+、N2H5+、C(NH2)3+ 和 N(CH3)4+ 的环戊唑酸盐的合成和表征
我们最近通过大量合成 (N5)6(H3O)3(NH4)4Cl 实现了多氮化学的突破,其中环五唑阴离子通过与 NH4+ 和 OH3+ 阳离子的氢桥广泛稳定。已经进行了大量努力以用能量更高的阳离子代替这些非能量阳离子和氯阴离子。本文报道了含有简单富氮阳离子 NH4+、NH3OH+、N2H5+、C(NH2)3+ 和 N(CH3)4+ 的环戊唑盐的复分解合成。这些盐的特征在于它们的晶体结构;振动、质量和多核 NMR 光谱;热稳定性测量;敏感性数据;和性能计算。结果表明,环戊唑酯比相应的叠氮化物更具能量,但在 80°C 至 105°C 的范围内分解时热稳定性较差。作为炸药,肼盐和羟铵盐预计与 RDX 的爆轰压力相匹配,但其爆速明显高于 RDX 和 HMX,具有相当的冲击和摩擦敏感性。虽然铵盐的爆轰压力比RDX低,但其爆速也超过了RDX和HMX。作为火箭推进剂,预计肼盐和羟铵盐的性能将超过 RDX 和 HMX。晶体结构表明,除了 N(CH3)4+ 盐也表现出强阳离子-π 相互作用外,环戊唑根阴离子通常通过与阳离子的氢键稳定。











































 京公网安备 11010802027423号
京公网安备 11010802027423号