Chemical Physics ( IF 2.0 ) Pub Date : 2018-11-02 , DOI: 10.1016/j.chemphys.2018.11.001 Lijuan Zhang , Dazhi Li
|
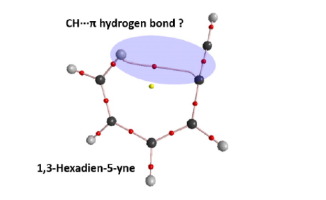
The blue-shifting intramolecular C–H···π hydrogen bonds (H-bonds) in conformer I of 1,3-hexadien-5-yne (13HD5Y) and its halogen-substituted derivatives are explored by theoretical calculations. The geometric analysis indicates that the C-H bond in conformer I is shortened in comparison with the non-interacting C-H bond, accompanied by the blue-shifts of the C-H stretching frequency. The formation of H-bonds is verified by the atoms-in-molecules and noncovalent interaction analysis. The H-bond energies are estimated to be -0.5∼-2.2 kcal·mol-1 by isodesmic reaction method at MP2/aug-cc-pVTZ level, which agree with the trends predicted by the geometrical and topological parameters. The results of the natural bond orbital analysis indicate that the origin of the blue-shifting H-bond may be due to the weak π(C≡C) → σ*(C–H) hyperconjugation interaction and the concomitant increasing in the s-character and polarization of C–H bond. This research provides a rare theoretical evidence to identify an intramolecular blue-shifting CH···π H-bond system.
中文翻译:

1,3-己二烯-5-炔及其卤素取代衍生物分子内蓝移CH··π氢键的研究
通过理论计算探索了1,3-己二烯-5-炔(13HD5Y)的构象异构体I中的蓝移分子内C–H··π氢键(H键)及其卤素取代的衍生物。几何分析表明,与非相互作用的CH键相比,构象异构体I中的CH键缩短了,伴随着CH拉伸频率的蓝移。通过分子中原子和非共价相互作用分析验证了H键的形成。在MP2 / aug-cc-pVTZ能级下,通过等渗反应法估计氢键能为-0.5〜-2.2 kcal·mol -1,与几何和拓扑参数预测的趋势一致。自然键轨道分析的结果表明,蓝移H键的起源可能是由于弱π(C≡C)→ σ *(C–H)超共轭相互作用,并且伴随s键和C–H键极化的增加。这项研究为鉴定分子内蓝移CH···πH键系统提供了罕见的理论依据。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号