当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Identification of Selective Dual ROCK1 and ROCK2 Inhibitors Using Structure-Based Drug Design
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2018-11-01 00:00:00 , DOI: 10.1021/acs.jmedchem.8b01098 Adrian D. Hobson 1 , Russell A. Judge 2 , Ana L. Aguirre 2 , Brian S. Brown 2 , Yifang Cui 3 , Ping Ding 1 , Eric Dominguez 1 , Enrico DiGiammarino 2 , David A. Egan 2 , Gail M. Freiberg 2 , Sujatha M. Gopalakrishnan 2 , Christopher M. Harris 1 , Marie P. Honore 2 , Karen L. Kage 2 , Nicolas J. Kapecki 2 , Christopher Ling 2 , Junli Ma 2 , Helmut Mack 3 , Mulugeta Mamo 2 , Stefan Maurus 3 , Bradford McRae 1 , Nigel S. Moore 1 , Bernhard K. Mueller 3 , Reinhold Mueller 3 , Marian T. Namovic 2 , Kaushal Patel 2 , Steve D. Pratt 2 , C. Brent Putman 2 , Kara L. Queeney 1 , Kathy K. Sarris 2 , Lisa M. Schaffter 1 , Vincent Stoll 2 , Anil Vasudevan 2 , Lei Wang 1 , Lu Wang 1 , William Wirthl 1 , Kimberly Yach 1
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2018-11-01 00:00:00 , DOI: 10.1021/acs.jmedchem.8b01098 Adrian D. Hobson 1 , Russell A. Judge 2 , Ana L. Aguirre 2 , Brian S. Brown 2 , Yifang Cui 3 , Ping Ding 1 , Eric Dominguez 1 , Enrico DiGiammarino 2 , David A. Egan 2 , Gail M. Freiberg 2 , Sujatha M. Gopalakrishnan 2 , Christopher M. Harris 1 , Marie P. Honore 2 , Karen L. Kage 2 , Nicolas J. Kapecki 2 , Christopher Ling 2 , Junli Ma 2 , Helmut Mack 3 , Mulugeta Mamo 2 , Stefan Maurus 3 , Bradford McRae 1 , Nigel S. Moore 1 , Bernhard K. Mueller 3 , Reinhold Mueller 3 , Marian T. Namovic 2 , Kaushal Patel 2 , Steve D. Pratt 2 , C. Brent Putman 2 , Kara L. Queeney 1 , Kathy K. Sarris 2 , Lisa M. Schaffter 1 , Vincent Stoll 2 , Anil Vasudevan 2 , Lei Wang 1 , Lu Wang 1 , William Wirthl 1 , Kimberly Yach 1
Affiliation
|
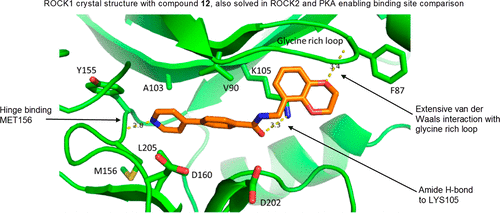
A HTS campaign identified compound 1, an excellent hit-like molecule to initiate medicinal chemistry efforts to optimize a dual ROCK1 and ROCK2 inhibitor. Substitution (2-Cl, 2-NH2, 2-F, 3-F) of the pyridine hinge binding motif or replacement with pyrimidine afforded compounds with a clean CYP inhibition profile. Cocrystal structures of an early lead compound were obtained in PKA, ROCK1, and ROCK2. This provided critical structural information for medicinal chemistry to drive compound design. The structural data indicated the preferred configuration at the central benzylic carbon would be (R), and application of this information to compound design resulted in compound 16. This compound was shown to be a potent and selective dual ROCK inhibitor in both enzyme and cell assays and efficacious in the retinal nerve fiber layer model after oral dosing. This tool compound has been made available through the AbbVie Compound Toolbox. Finally, the cocrystal structures also identified that aspartic acid residues 176 and 218 in ROCK2, which are glutamic acids in PKA, could be targeted as residues to drive both potency and kinome selectivity. Introduction of a piperidin-3-ylmethanamine group to the compound series resulted in compound 58, a potent and selective dual ROCK inhibitor with excellent predicted drug-like properties.
中文翻译:

基于结构的药物设计鉴定选择性双重ROCK1和ROCK2抑制剂
一项HTS运动确定了化合物1,这是一种出色的命中分子,可启动药物化学工作以优化ROCK1和ROCK2双重抑制剂。取代吡啶铰链结合基序的(2-Cl,2-NH 2,2 -F,3-F)或用嘧啶替代,提供了具有干净CYP抑制谱的化合物。在PKA,ROCK1和ROCK2中获得了早期铅化合物的共晶体结构。这为药物化学驱动化合物设计提供了关键的结构信息。结构数据表明,中心苄基碳原子的优选构型为(R),并且将该信息应用于化合物设计可得到化合物16。该化合物在酶和细胞分析中均显示为有效且选择性的双重ROCK抑制剂,口服给药后在视网膜神经纤维层模型中有效。该工具化合物可通过AbbVie Compound Toolbox获得。最后,共晶结构还确定了ROCK2中的天冬氨酸残基176和218(它们是PKA中的谷氨酸)可以作为残基来靶向,以同时驱动效价和激酶组选择性。在化合物系列中引入哌啶-3-氨基甲胺基团产生了化合物58,这是一种有效且选择性的双重ROCK抑制剂,具有出色的预测类药物特性。
更新日期:2018-11-01
中文翻译:

基于结构的药物设计鉴定选择性双重ROCK1和ROCK2抑制剂
一项HTS运动确定了化合物1,这是一种出色的命中分子,可启动药物化学工作以优化ROCK1和ROCK2双重抑制剂。取代吡啶铰链结合基序的(2-Cl,2-NH 2,2 -F,3-F)或用嘧啶替代,提供了具有干净CYP抑制谱的化合物。在PKA,ROCK1和ROCK2中获得了早期铅化合物的共晶体结构。这为药物化学驱动化合物设计提供了关键的结构信息。结构数据表明,中心苄基碳原子的优选构型为(R),并且将该信息应用于化合物设计可得到化合物16。该化合物在酶和细胞分析中均显示为有效且选择性的双重ROCK抑制剂,口服给药后在视网膜神经纤维层模型中有效。该工具化合物可通过AbbVie Compound Toolbox获得。最后,共晶结构还确定了ROCK2中的天冬氨酸残基176和218(它们是PKA中的谷氨酸)可以作为残基来靶向,以同时驱动效价和激酶组选择性。在化合物系列中引入哌啶-3-氨基甲胺基团产生了化合物58,这是一种有效且选择性的双重ROCK抑制剂,具有出色的预测类药物特性。































 京公网安备 11010802027423号
京公网安备 11010802027423号