当前位置:
X-MOL 学术
›
Acta Biomater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A stabilized retro-inverso peptide ligand of transferrin receptor for enhanced liposome-based hepatocellular carcinoma-targeted drug delivery.
Acta Biomaterialia ( IF 9.4 ) Pub Date : 2018-11-03 , DOI: 10.1016/j.actbio.2018.11.002 Jiajing Tang 1 , Qiantao Wang 1 , Qianwen Yu 1 , Yue Qiu 1 , Ling Mei 1 , Dandan Wan 1 , Xuhui Wang 1 , Man Li 1 , Qin He 1
Acta Biomaterialia ( IF 9.4 ) Pub Date : 2018-11-03 , DOI: 10.1016/j.actbio.2018.11.002 Jiajing Tang 1 , Qiantao Wang 1 , Qianwen Yu 1 , Yue Qiu 1 , Ling Mei 1 , Dandan Wan 1 , Xuhui Wang 1 , Man Li 1 , Qin He 1
Affiliation
|
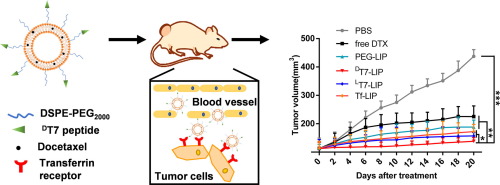
The application of tumor targeting ligands to the treatment of cancer holds promise for improving efficacy and reducing toxicity. LT7 (L(HAIYPRH)) peptide, a phage display-selected peptide, exhibited high binding affinity to transferrin receptor (TfR) overexpressed on tumor cells. However, its in vivo tumor targeting efficiency was impaired due to enzymatic degradation in blood circulation. To improve the stability and targeting ability, a retro-inverso analogue of LT7 peptide, named DT7 peptide (D(HRPYIAH)), was designed for targeted therapy of hepatocellular carcinoma. The result of computer simulation predicted that DT7 bound to TfR protein more efficiently than LT7, and this prediction was confirmed experimentally by surface plasmon resonance (SPR). Ex vivo stability experiment demonstrated that DT7 possessed stronger ability against proteolysis than LT7 in fresh mouse serum. We further prepared DT7-, LT7-, and transferrin (Tf)-modified liposomes (DT7-LIP, LT7-LIP, and Tf-LIP, respectively). DT7-LIP showed a significantly stronger in vitro targeting ability than LT7-LIP and Tf-LIP under normal condition and simulated biological condition. In addition, the in vitro antitumor effect of DTX-loaded DT7-LIP was markedly enhanced in comparison to DTX-loaded LT7-LIP and DTX-loaded Tf-LIP. In vivo imaging indicated that DT7-LIP had better tumor accumulation than LT7-LIP and Tf-LIP. For in vivo antitumor studies, the tumor growth rate of mice treated with DTX-loaded DT7-LIP was significantly inhibited compared to that in mice treated with DTX-loaded LT7-LIP and DTX-loaded Tf-LIP. Overall, this study verified the potential of the stable DT7 peptide in improving the efficacy of docetaxel in the treatment of hepatocellular carcinoma. STATEMENT OF SIGNIFICANCE: A phage display library-selected LT7 (L(HAIYPRH)) peptide exhibited high affinity to transferrin receptor (TfR). However, its bioactivity was impaired in vivo as L-peptides are susceptible to degradation by proteolytic enzymes. Here, we designed a retro-inverso peptide DT7(D(HRPYIAH)) and demonstrated its increased serum stability and higher binding affinity to TfR. A stabilized targeted drug delivery system was further constructed by modified DT7 peptide on the surface of liposomes. The data indicated that DT7 peptide-modified liposomes exhibited higher targeting ability in vitro and in vivo. More importantly, DT7-modified liposomes demonstrated positive preclinical significance in enhancing the therapeutic effects against hepatocellular carcinoma.
中文翻译:

转铁蛋白受体的稳定逆反肽配体,用于增强基于脂质体的肝细胞癌靶向药物的递送。
肿瘤靶向配体在癌症治疗中的应用有望改善疗效并降低毒性。LT7(L(HAIYPRH))肽,一种噬菌体展示选择的肽,与在肿瘤细胞上过表达的转铁蛋白受体(TfR)表现出高结合亲和力。然而,由于血液循环中的酶促降解,其体内肿瘤靶向效率受到损害。为了提高稳定性和靶向能力,设计了一种称为DT7肽(D(HRPYIAH))的LT7肽逆向类似物,用于肝细胞癌的靶向治疗。计算机模拟的结果表明,DT7比LT7更有效地与TfR蛋白结合,这一预测已通过表面等离振子共振(SPR)进行了实验证实。离体稳定性实验表明,在新鲜小鼠血清中,DT7具有比LT7更强的蛋白水解能力。我们进一步制备了DT7,LT7和转铁蛋白(Tf)修饰的脂质体(分别为DT7-LIP,LT7-LIP和Tf-LIP)。在正常条件和模拟生物学条件下,DT7-LIP的体外靶向能力比LT7-LIP和Tf-LIP显着强。此外,与DTX加载的LT7-LIP和DTX加载的Tf-LIP相比,DTX加载的DT7-LIP的体外抗肿瘤作用显着增强。体内成像表明,DT7-LIP比LT7-LIP和Tf-LIP具有更好的肿瘤蓄积性。对于体内抗肿瘤研究,与用DTX加载的LT7-LIP和DTX加载的Tf-LIP处理的小鼠相比,用DTX加载的DT7-LIP处理的小鼠的肿瘤生长速度受到显着抑制。全面的,这项研究证实了稳定的DT7肽在提高多西紫杉醇治疗肝细胞癌疗效方面的潜力。重要性说明:噬菌体展示库选择的LT7(L(HAIYPRH))肽对转铁蛋白受体(TfR)表现出高亲和力。然而,由于L-肽易于被蛋白水解酶降解,因此其生物活性在体内受到损害。在这里,我们设计了逆向逆转录肽DT7(D(HRPYIAH)),并证明了其提高的血清稳定性和对TfR的更高结合亲和力。通过修饰的DT7肽在脂质体表面上进一步构建了稳定的靶向药物递送系统。数据表明,DT7肽修饰的脂质体在体外和体内表现出更高的靶向能力。更重要的是,
更新日期:2018-11-05
中文翻译:

转铁蛋白受体的稳定逆反肽配体,用于增强基于脂质体的肝细胞癌靶向药物的递送。
肿瘤靶向配体在癌症治疗中的应用有望改善疗效并降低毒性。LT7(L(HAIYPRH))肽,一种噬菌体展示选择的肽,与在肿瘤细胞上过表达的转铁蛋白受体(TfR)表现出高结合亲和力。然而,由于血液循环中的酶促降解,其体内肿瘤靶向效率受到损害。为了提高稳定性和靶向能力,设计了一种称为DT7肽(D(HRPYIAH))的LT7肽逆向类似物,用于肝细胞癌的靶向治疗。计算机模拟的结果表明,DT7比LT7更有效地与TfR蛋白结合,这一预测已通过表面等离振子共振(SPR)进行了实验证实。离体稳定性实验表明,在新鲜小鼠血清中,DT7具有比LT7更强的蛋白水解能力。我们进一步制备了DT7,LT7和转铁蛋白(Tf)修饰的脂质体(分别为DT7-LIP,LT7-LIP和Tf-LIP)。在正常条件和模拟生物学条件下,DT7-LIP的体外靶向能力比LT7-LIP和Tf-LIP显着强。此外,与DTX加载的LT7-LIP和DTX加载的Tf-LIP相比,DTX加载的DT7-LIP的体外抗肿瘤作用显着增强。体内成像表明,DT7-LIP比LT7-LIP和Tf-LIP具有更好的肿瘤蓄积性。对于体内抗肿瘤研究,与用DTX加载的LT7-LIP和DTX加载的Tf-LIP处理的小鼠相比,用DTX加载的DT7-LIP处理的小鼠的肿瘤生长速度受到显着抑制。全面的,这项研究证实了稳定的DT7肽在提高多西紫杉醇治疗肝细胞癌疗效方面的潜力。重要性说明:噬菌体展示库选择的LT7(L(HAIYPRH))肽对转铁蛋白受体(TfR)表现出高亲和力。然而,由于L-肽易于被蛋白水解酶降解,因此其生物活性在体内受到损害。在这里,我们设计了逆向逆转录肽DT7(D(HRPYIAH)),并证明了其提高的血清稳定性和对TfR的更高结合亲和力。通过修饰的DT7肽在脂质体表面上进一步构建了稳定的靶向药物递送系统。数据表明,DT7肽修饰的脂质体在体外和体内表现出更高的靶向能力。更重要的是,

































 京公网安备 11010802027423号
京公网安备 11010802027423号