当前位置:
X-MOL 学术
›
ChemistrySelect
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Biphenyl 3‐ and 4‐ mono and 4, 4′‐dicarbaldehyde as Electrophiles and Unusual Michael Acceptors in the Baylis‐Hillman Reaction: Synthesis of Functionalized Biphenyl Derivatives
ChemistrySelect ( IF 1.9 ) Pub Date : 2018-11-02 , DOI: 10.1002/slct.201802770 Mohanakumaran Athira 1 , Ramakrishnan Suseela Meerakrishna 1 , Ponnusamy Shanmugam 1
ChemistrySelect ( IF 1.9 ) Pub Date : 2018-11-02 , DOI: 10.1002/slct.201802770 Mohanakumaran Athira 1 , Ramakrishnan Suseela Meerakrishna 1 , Ponnusamy Shanmugam 1
Affiliation
|
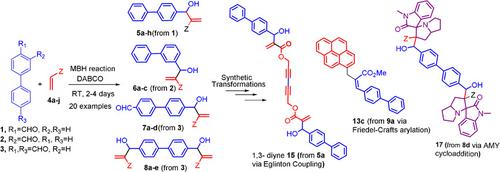
The Morita‐Baylis‐Hillman (MBH) reaction of biphenyl 3‐ and 4‐ mono aldehydes and 4, 4′‐dicarbaldehyde as electrophiles and a number of unexplored activated alkenes afforded highly functionalized biphenyl derivatives in excellent yield. Synthetic transformations of mono and bis MBH adducts thus obtained have been demonstrated by isomerization of MBH adducts with different nucleophiles, Friedel‐Crafts arylation reaction of bromo isomerized biphenyl adducts, Eglinton coupling of biphenyl propargyl MBH adduct derivative and [3+2]‐ azomethine ylide cycloaddition of methyl acrylate derived biphenyl aldehydes afforded highly functionalized arylated and heterocyclic derivatives in very good yield.
中文翻译:

联苯3-和4-单和4,4'-二甲醛作为亲电子试剂和不常见的Michael受体在Baylis-Hillman反应中:官能化联苯衍生物的合成
联苯3-和4-单醛和4,4'-二甲醛作为亲电子试剂和许多未开发的活化烯烃的Morita-Baylis-Hillman(MBH)反应以优异的收率提供了高度官能化的联苯衍生物。如此获得的单和双MBH加合物的合成转化已通过具有不同亲核试剂的MBH加合物的异构化,溴代异构化的联苯加合物的Friedel-Crafts芳基化反应,联苯基炔丙基MBH加合物衍生物和[3 + 2]-偶氮甲亚胺基内酯的Eglinton偶联来证明丙烯酸甲酯衍生的联苯醛的环加成反应以非常好的收率提供了高度官能化的芳基化和杂环衍生物。
更新日期:2018-11-02
中文翻译:

联苯3-和4-单和4,4'-二甲醛作为亲电子试剂和不常见的Michael受体在Baylis-Hillman反应中:官能化联苯衍生物的合成
联苯3-和4-单醛和4,4'-二甲醛作为亲电子试剂和许多未开发的活化烯烃的Morita-Baylis-Hillman(MBH)反应以优异的收率提供了高度官能化的联苯衍生物。如此获得的单和双MBH加合物的合成转化已通过具有不同亲核试剂的MBH加合物的异构化,溴代异构化的联苯加合物的Friedel-Crafts芳基化反应,联苯基炔丙基MBH加合物衍生物和[3 + 2]-偶氮甲亚胺基内酯的Eglinton偶联来证明丙烯酸甲酯衍生的联苯醛的环加成反应以非常好的收率提供了高度官能化的芳基化和杂环衍生物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号