Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Metal-free ribonucleotide reduction powered by a DOPA radical in Mycoplasma pathogens
Nature ( IF 50.5 ) Pub Date : 2018-10-31 , DOI: 10.1038/s41586-018-0653-6 Vivek Srinivas 1 , Hugo Lebrette 1 , Daniel Lundin 1 , Yuri Kutin 2 , Margareta Sahlin 1 , Michael Lerche 1 , Jürgen Eirich 3 , Rui M M Branca 3 , Nicholas Cox 4 , Britt-Marie Sjöberg 1 , Martin Högbom 1, 5
Nature ( IF 50.5 ) Pub Date : 2018-10-31 , DOI: 10.1038/s41586-018-0653-6 Vivek Srinivas 1 , Hugo Lebrette 1 , Daniel Lundin 1 , Yuri Kutin 2 , Margareta Sahlin 1 , Michael Lerche 1 , Jürgen Eirich 3 , Rui M M Branca 3 , Nicholas Cox 4 , Britt-Marie Sjöberg 1 , Martin Högbom 1, 5
Affiliation
|
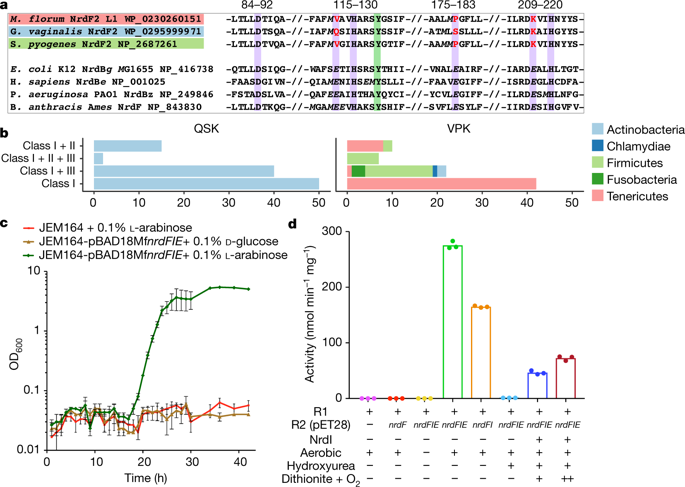
Ribonucleotide reductase (RNR) catalyses the only known de novo pathway for the production of all four deoxyribonucleotides that are required for DNA synthesis1,2. It is essential for all organisms that use DNA as their genetic material and is a current drug target3,4. Since the discovery that iron is required for function in the aerobic, class I RNR found in all eukaryotes and many bacteria, a dinuclear metal site has been viewed as necessary to generate and stabilize the catalytic radical that is essential for RNR activity5–7. Here we describe a group of RNR proteins in Mollicutes—including Mycoplasma pathogens—that possess a metal-independent stable radical residing on a modified tyrosyl residue. Structural, biochemical and spectroscopic characterization reveal a stable 3,4-dihydroxyphenylalanine (DOPA) radical species that directly supports ribonucleotide reduction in vitro and in vivo. This observation overturns the presumed requirement for a dinuclear metal site in aerobic ribonucleotide reductase. The metal-independent radical requires new mechanisms for radical generation and stabilization, processes that are targeted by RNR inhibitors. It is possible that this RNR variant provides an advantage under metal starvation induced by the immune system. Organisms that encode this type of RNR—some of which are developing resistance to antibiotics—are involved in diseases of the respiratory, urinary and genital tracts. Further characterization of this RNR family and its mechanism of cofactor generation will provide insight into new enzymatic chemistry and be of value in devising strategies to combat the pathogens that utilize it. We propose that this RNR subclass is denoted class Ie.A subclass of ribonucleotide reductase in Mycoplasma pathogens contains a stable radical formed from a modified tyrosine residue, overturning the presumed requirement for a dinuclear metal site in aerobic ribonucleotide reductase.
中文翻译:

支原体病原体中多巴自由基驱动的无金属核糖核苷酸还原
核糖核苷酸还原酶 (RNR) 催化唯一已知的从头途径产生 DNA 合成所需的所有四种脱氧核糖核苷酸 1,2。它对于所有使用 DNA 作为遗传物质的生物体都是必不可少的,并且是当前的药物靶点3,4。自从发现铁是所有真核生物和许多细菌中的有氧 I 类 RNR 功能所必需的,双核金属位点被认为是产生和稳定 RNR 活性所必需的催化自由基所必需的 5-7。在这里,我们描述了软体动物(包括支原体病原体)中的一组 RNR 蛋白,它们在修饰的酪氨酰残基上具有不依赖于金属的稳定自由基。结构、生化和光谱表征揭示了稳定的 3,4-二羟基苯丙氨酸 (DOPA) 自由基种类,可直接支持体外和体内核糖核苷酸还原。这一观察结果推翻了需氧核糖核苷酸还原酶中双核金属位点的假定要求。不依赖于金属的自由基需要新的自由基产生和稳定机制,这些过程是 RNR 抑制剂的目标。这种 RNR 变体可能在免疫系统诱导的金属饥饿下提供优势。编码此类 RNR 的生物体(其中一些正在对抗生素产生耐药性)与呼吸道、泌尿道和生殖道疾病有关。该 RNR 家族及其辅因子生成机制的进一步表征将提供对新酶化学的深入了解,并且对于制定对抗利用它的病原体的策略具有价值。我们建议该 RNR 亚类表示为 Ie 类。支原体病原体中的核糖核苷酸还原酶亚类包含由修饰的酪氨酸残基形成的稳定自由基,推翻了需氧核糖核苷酸还原酶中双核金属位点的假定要求。
更新日期:2018-10-31
中文翻译:

支原体病原体中多巴自由基驱动的无金属核糖核苷酸还原
核糖核苷酸还原酶 (RNR) 催化唯一已知的从头途径产生 DNA 合成所需的所有四种脱氧核糖核苷酸 1,2。它对于所有使用 DNA 作为遗传物质的生物体都是必不可少的,并且是当前的药物靶点3,4。自从发现铁是所有真核生物和许多细菌中的有氧 I 类 RNR 功能所必需的,双核金属位点被认为是产生和稳定 RNR 活性所必需的催化自由基所必需的 5-7。在这里,我们描述了软体动物(包括支原体病原体)中的一组 RNR 蛋白,它们在修饰的酪氨酰残基上具有不依赖于金属的稳定自由基。结构、生化和光谱表征揭示了稳定的 3,4-二羟基苯丙氨酸 (DOPA) 自由基种类,可直接支持体外和体内核糖核苷酸还原。这一观察结果推翻了需氧核糖核苷酸还原酶中双核金属位点的假定要求。不依赖于金属的自由基需要新的自由基产生和稳定机制,这些过程是 RNR 抑制剂的目标。这种 RNR 变体可能在免疫系统诱导的金属饥饿下提供优势。编码此类 RNR 的生物体(其中一些正在对抗生素产生耐药性)与呼吸道、泌尿道和生殖道疾病有关。该 RNR 家族及其辅因子生成机制的进一步表征将提供对新酶化学的深入了解,并且对于制定对抗利用它的病原体的策略具有价值。我们建议该 RNR 亚类表示为 Ie 类。支原体病原体中的核糖核苷酸还原酶亚类包含由修饰的酪氨酸残基形成的稳定自由基,推翻了需氧核糖核苷酸还原酶中双核金属位点的假定要求。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号