当前位置:
X-MOL 学术
›
Am. J. Hum. Genet.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Germline De Novo Mutations in ATP1A1 Cause Renal Hypomagnesemia, Refractory Seizures, and Intellectual Disability.
American Journal of Human Genetics ( IF 8.1 ) Pub Date : 2018-11-01 , DOI: 10.1016/j.ajhg.2018.10.004 Karl P Schlingmann 1 , Sascha Bandulik 2 , Cherry Mammen 3 , Maja Tarailo-Graovac 4 , Rikke Holm 5 , Matthias Baumann 6 , Jens König 1 , Jessica J Y Lee 7 , Britt Drögemöller 7 , Katrin Imminger 2 , Bodo B Beck 8 , Janine Altmüller 9 , Holger Thiele 9 , Siegfried Waldegger 6 , William Van't Hoff 10 , Robert Kleta 11 , Richard Warth 2 , Clara D M van Karnebeek 12 , Bente Vilsen 5 , Detlef Bockenhauer 11 , Martin Konrad 1
American Journal of Human Genetics ( IF 8.1 ) Pub Date : 2018-11-01 , DOI: 10.1016/j.ajhg.2018.10.004 Karl P Schlingmann 1 , Sascha Bandulik 2 , Cherry Mammen 3 , Maja Tarailo-Graovac 4 , Rikke Holm 5 , Matthias Baumann 6 , Jens König 1 , Jessica J Y Lee 7 , Britt Drögemöller 7 , Katrin Imminger 2 , Bodo B Beck 8 , Janine Altmüller 9 , Holger Thiele 9 , Siegfried Waldegger 6 , William Van't Hoff 10 , Robert Kleta 11 , Richard Warth 2 , Clara D M van Karnebeek 12 , Bente Vilsen 5 , Detlef Bockenhauer 11 , Martin Konrad 1
Affiliation
|
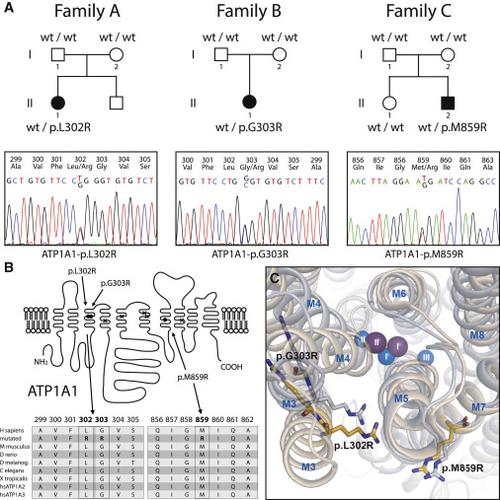
Over the last decades, a growing spectrum of monogenic disorders of human magnesium homeostasis has been clinically characterized, and genetic studies in affected individuals have identified important molecular components of cellular and epithelial magnesium transport. Here, we describe three infants who are from non-consanguineous families and who presented with a disease phenotype consisting of generalized seizures in infancy, severe hypomagnesemia, and renal magnesium wasting. Seizures persisted despite magnesium supplementation and were associated with significant intellectual disability. Whole-exome sequencing and conventional Sanger sequencing identified heterozygousde novomutations in the catalytic Na+, K+-ATPase α1 subunit (ATP1A1). Functional characterization of mutant Na+, K+-ATPase α1 subunits in heterologous expression systems revealed not only a loss of Na+, K+-ATPase function but also abnormal cation permeabilities, which led to membrane depolarization and possibly aggravated the effect of the loss of physiological pump activity. These findings underline the indispensable role of the α1 isoform of the Na+, K+-ATPase for renal-tubular magnesium handling and cellular ion homeostasis, as well as maintenance of physiologic neuronal activity.
中文翻译:

ATP1A1中的种胚从头突变会导致肾低镁血症,难治性癫痫发作和智力障碍。
在过去的几十年中,人类镁稳态的单基因疾病谱已在临床上得到表征,并且对受影响个体的遗传研究已经确定了细胞和上皮镁转运的重要分子成分。在这里,我们描述了三个来自非近亲家庭的婴儿,他们表现出一种疾病表型,包括婴儿期普遍性癫痫发作,严重低镁血症和肾镁消耗。尽管补充了镁,癫痫发作仍然持续,并且与严重的智力障碍有关。全外显子测序和常规Sanger测序确定了催化Na +,K + -ATPaseα1亚基(ATP1A1)中的杂合novomutation。突变体Na +的功能表征 异源表达系统中的K + -ATPaseα1亚基不仅显示Na +,K + -ATPase功能丧失,而且阳离子通透性异常,这导致膜去极化,并可能加剧生理泵活性丧失的影响。这些发现强调了Na +,K + -ATPase的α1亚型在肾小管镁处理和细胞离子稳态以及维持生理神经元活动中不可或缺的作用。
更新日期:2018-11-02
中文翻译:

ATP1A1中的种胚从头突变会导致肾低镁血症,难治性癫痫发作和智力障碍。
在过去的几十年中,人类镁稳态的单基因疾病谱已在临床上得到表征,并且对受影响个体的遗传研究已经确定了细胞和上皮镁转运的重要分子成分。在这里,我们描述了三个来自非近亲家庭的婴儿,他们表现出一种疾病表型,包括婴儿期普遍性癫痫发作,严重低镁血症和肾镁消耗。尽管补充了镁,癫痫发作仍然持续,并且与严重的智力障碍有关。全外显子测序和常规Sanger测序确定了催化Na +,K + -ATPaseα1亚基(ATP1A1)中的杂合novomutation。突变体Na +的功能表征 异源表达系统中的K + -ATPaseα1亚基不仅显示Na +,K + -ATPase功能丧失,而且阳离子通透性异常,这导致膜去极化,并可能加剧生理泵活性丧失的影响。这些发现强调了Na +,K + -ATPase的α1亚型在肾小管镁处理和细胞离子稳态以及维持生理神经元活动中不可或缺的作用。






































 京公网安备 11010802027423号
京公网安备 11010802027423号