当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Neolymphostin A Is a Covalent Phosphoinositide 3-Kinase (PI3K)/Mammalian Target of Rapamycin (mTOR) Dual Inhibitor That Employs an Unusual Electrophilic Vinylogous Ester.
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2018-11-28 , DOI: 10.1021/acs.jmedchem.8b00975 Gabriel Castro-Falcón 1 , Grant S Seiler 1, 2 , Özlem Demir 2 , Manoj K Rathinaswamy 3 , David Hamelin 3 , Reece M Hoffmann 3 , Stefanie L Makowski 4 , Anne-Catrin Letzel 1 , Seth J Field 4 , John E Burke 3 , Rommie E Amaro 2 , Chambers C Hughes 1
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2018-11-28 , DOI: 10.1021/acs.jmedchem.8b00975 Gabriel Castro-Falcón 1 , Grant S Seiler 1, 2 , Özlem Demir 2 , Manoj K Rathinaswamy 3 , David Hamelin 3 , Reece M Hoffmann 3 , Stefanie L Makowski 4 , Anne-Catrin Letzel 1 , Seth J Field 4 , John E Burke 3 , Rommie E Amaro 2 , Chambers C Hughes 1
Affiliation
|
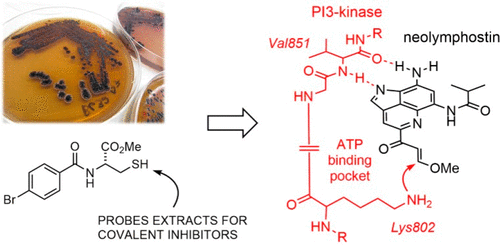
Using a novel chemistry-based assay for identifying electrophilic natural products in unprocessed extracts, we identified the PI3-kinase/mTOR dual inhibitor neolymphostin A from Salinispora arenicola CNY-486. The method further showed that the vinylogous ester substituent on the neolymphostin core was the exact site for enzyme conjugation. Tandem MS/MS experiments on PI3Kα treated with the inhibitor revealed that neolymphostin covalently modified Lys802 with a shift in mass of +306 amu, corresponding to addition of the inhibitor and elimination of methanol. The binding pose of the inhibitor bound to PI3Kα was modeled, and hydrogen-deuterium exchange mass spectrometry experiments supported this model. Against a panel of kinases, neolymphostin showed good selectivity for PI3-kinase and mTOR. In addition, the natural product blocked AKT phosphorylation in live cells with an IC50 of ∼3 nM. Taken together, neolymphostin is the first reported example of a covalent kinase inhibitor from the bacterial domain of life.
中文翻译:

Neolymphostin A 是一种共价磷酸肌醇 3-激酶 (PI3K)/雷帕霉素哺乳动物靶标 (mTOR) 双重抑制剂,采用不寻常的亲电乙烯酯。
使用一种基于化学的新型测定法来鉴定未加工提取物中的亲电子天然产物,我们从 Salinispora arenicola CNY-486 中鉴定出 PI3 激酶/mTOR 双重抑制剂新淋巴素 A。该方法进一步表明新淋巴素核心上的插烯酯取代基是酶缀合的确切位点。对用抑制剂处理的 PI3Kα 进行的串联 MS/MS 实验表明,新淋巴素共价修饰了 Lys802,质量发生 +306 amu 的变化,对应于抑制剂的添加和甲醇的消除。对抑制剂与 PI3Kα 结合的结合姿势进行了建模,氢-氘交换质谱实验支持了该模型。针对一组激酶,新淋巴素对 PI3 激酶和 mTOR 显示出良好的选择性。此外,该天然产物还能阻断活细胞中的 AKT 磷酸化,IC50 为 ∼3 nM。总而言之,新淋巴素是第一个报道的来自细菌生命领域的共价激酶抑制剂的例子。
更新日期:2018-10-31
中文翻译:

Neolymphostin A 是一种共价磷酸肌醇 3-激酶 (PI3K)/雷帕霉素哺乳动物靶标 (mTOR) 双重抑制剂,采用不寻常的亲电乙烯酯。
使用一种基于化学的新型测定法来鉴定未加工提取物中的亲电子天然产物,我们从 Salinispora arenicola CNY-486 中鉴定出 PI3 激酶/mTOR 双重抑制剂新淋巴素 A。该方法进一步表明新淋巴素核心上的插烯酯取代基是酶缀合的确切位点。对用抑制剂处理的 PI3Kα 进行的串联 MS/MS 实验表明,新淋巴素共价修饰了 Lys802,质量发生 +306 amu 的变化,对应于抑制剂的添加和甲醇的消除。对抑制剂与 PI3Kα 结合的结合姿势进行了建模,氢-氘交换质谱实验支持了该模型。针对一组激酶,新淋巴素对 PI3 激酶和 mTOR 显示出良好的选择性。此外,该天然产物还能阻断活细胞中的 AKT 磷酸化,IC50 为 ∼3 nM。总而言之,新淋巴素是第一个报道的来自细菌生命领域的共价激酶抑制剂的例子。































 京公网安备 11010802027423号
京公网安备 11010802027423号