当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The O-Carbamoyl-Transferase Alb15 Is Responsible for the Modification of Albicidin
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2016-02-23 00:00:00 , DOI: 10.1021/acschembio.5b01001 Daniel Petras 1 , Dennis Kerwat 1 , Alexander Pesic 1 , Benjamin-F Hempel 1 , Leonard von Eckardstein 1 , Siamak Semsary 1 , Julie Arasté 2 , Mélanie Marguerettaz 2 , Monique Royer 2 , Stéphane Cociancich 2 , Roderich D. Süssmuth 1
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2016-02-23 00:00:00 , DOI: 10.1021/acschembio.5b01001 Daniel Petras 1 , Dennis Kerwat 1 , Alexander Pesic 1 , Benjamin-F Hempel 1 , Leonard von Eckardstein 1 , Siamak Semsary 1 , Julie Arasté 2 , Mélanie Marguerettaz 2 , Monique Royer 2 , Stéphane Cociancich 2 , Roderich D. Süssmuth 1
Affiliation
|
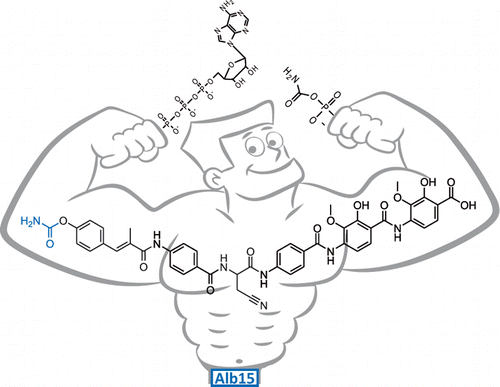
Albicidin is a potent antibiotic and phytotoxin produced by Xanthomonas albilineans which targets the plant and bacterial DNA gyrase. We now report on a new albicidin derivative which is carbamoylated at the N-terminal coumaric acid by the action of the ATP-dependent O-carbamoyltransferase Alb15, present in the albicidin (alb) gene cluster. Carbamoyl-albicidin was characterized by tandem mass spectrometry from cultures of a Xanthomonas overproducer strain and the gene function confirmed by gene inactivation of alb15 in X. albilineans. Expression of alb15 in Escherichia coli and in vitro reconstitution of the carbamoyltransferase activity confirmed albicidin as the substrate. The chemical synthesis of carbamoyl-albicidin finally enabled us to assess its bioactivity by means of in vitro gyrase inhibition and antibacterial assays. Compared to albicidin, carbamoyl-albicidin showed a significantly higher inhibitory efficiency against bacterial gyrase (∼8 vs 49 nM), which identifies the carbamoyl group as an important structural feature of albicidin maturation.
中文翻译:

该Ø氨甲酰基转移Alb15负责的albicidin的修改
阿比西丁是一种有效的抗生素和植物毒素,由黄单胞菌(Xanthomonas albilineans)产生,靶向植物和细菌DNA促旋酶。现在,我们报告了一种新的茜素衍生物,该衍生物在N-末端香豆酸的氨基甲酰化反应中,通过ATP依赖性O-氨基甲酰基转移酶Alb15的作用存在于茜素(alb)基因簇中。通过串联质谱法从黄单胞菌高产菌株的培养物中表征氨基甲酰-阿比西丁,并通过在白僵线霉中alb15的基因失活证实了基因功能。的表达alb15在大肠杆菌和体外氨基甲酰基转移酶活性的重建证实了阿比西丁为底物。氨基甲酰基阿比西丁的化学合成最终使我们能够通过体外促旋酶抑制和抗菌测定评估其生物活性。与阿比西丁相比,氨基甲酰基-阿比西丁对细菌促旋酶的抑制作用明显更高(〜8 vs 49 nM),这表明氨基甲酰基是阿比西丁成熟的重要结构特征。
更新日期:2016-02-23
中文翻译:

该Ø氨甲酰基转移Alb15负责的albicidin的修改
阿比西丁是一种有效的抗生素和植物毒素,由黄单胞菌(Xanthomonas albilineans)产生,靶向植物和细菌DNA促旋酶。现在,我们报告了一种新的茜素衍生物,该衍生物在N-末端香豆酸的氨基甲酰化反应中,通过ATP依赖性O-氨基甲酰基转移酶Alb15的作用存在于茜素(alb)基因簇中。通过串联质谱法从黄单胞菌高产菌株的培养物中表征氨基甲酰-阿比西丁,并通过在白僵线霉中alb15的基因失活证实了基因功能。的表达alb15在大肠杆菌和体外氨基甲酰基转移酶活性的重建证实了阿比西丁为底物。氨基甲酰基阿比西丁的化学合成最终使我们能够通过体外促旋酶抑制和抗菌测定评估其生物活性。与阿比西丁相比,氨基甲酰基-阿比西丁对细菌促旋酶的抑制作用明显更高(〜8 vs 49 nM),这表明氨基甲酰基是阿比西丁成熟的重要结构特征。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号