当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Carboxylic Acid-Promoted Single-Step Indole Construction from Simple Anilines and Ketones via Aerobic Cross-Dehydrogenative Coupling
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2018-10-31 00:00:00 , DOI: 10.1021/acs.joc.8b02180 Long Ren 1 , Guanglei Nan 1 , Yongcheng Wang 1 , Zhiyan Xiao 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2018-10-31 00:00:00 , DOI: 10.1021/acs.joc.8b02180 Long Ren 1 , Guanglei Nan 1 , Yongcheng Wang 1 , Zhiyan Xiao 1
Affiliation
|
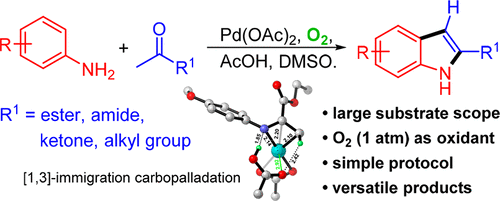
The cross-dehydrogenative coupling (CDC) reaction is an efficient strategy for indole synthesis. However, most CDC methods require special substrates, and the presence of inherent groups limits the versatility for further transformation. A carboxylic acid-promoted aerobic catalytic system is developed herein for a single-step synthesis of indoles from simple anilines and ketones. This versatile system is featured by the broad substrate scope and the use of ambient oxygen as an oxidant and is convenient and economical for both laboratory and industry applications. The existence of the labile hydrogen at C-3 and the highly transformable carbonyl at C-2 makes the indoles versatile building blocks for organic synthesis in different contexts. Computational studies based on the density functional theory (DFT) suggest that the rate-determining step is carboxylic acid-assisted condensation of the substrates, rather than the functionalization of aryl C–H. Accordingly, a pathway via imine intermediates is deemed to be the preferred mechanism. In contrast to the general deduction, the in situ formed imine, instead of its enamine isomer, is believed to be involved in the first ligand exchange and later carbopalladation of the α-Me, which shed new light on this indolization mechanism.
中文翻译:

通过有氧交叉脱氢偶联从简单的苯胺和酮中羧酸促进的一步吲哚构建
交叉脱氢偶联(CDC)反应是吲哚合成的有效策略。但是,大多数CDC方法需要特殊的底物,并且固有基团的存在限制了进一步转化的通用性。本文开发了一种羧酸促进的需氧催化系统,用于由简单的苯胺和酮一步合成吲哚。这种多功能的系统具有广泛的底物范围和使用环境氧气作为氧化剂的特点,对于实验室和工业应用均既方便又经济。C-3上不稳定的氢和C-2上高度可转化的羰基的存在,使吲哚在各种情况下成为有机合成的通用结构单元。基于密度泛函理论(DFT)的计算研究表明,决定速率的步骤是底物的羧酸辅助缩合,而不是芳基CH的官能化。因此,经由亚胺中间体的途径被认为是优选的机制。与一般的推论相反,据信原位形成的亚胺代替其烯胺异构体参与了α-Me的首次配体交换和随后的碳pal化,这为这种吲哚化机理提供了新的思路。
更新日期:2018-10-31
中文翻译:

通过有氧交叉脱氢偶联从简单的苯胺和酮中羧酸促进的一步吲哚构建
交叉脱氢偶联(CDC)反应是吲哚合成的有效策略。但是,大多数CDC方法需要特殊的底物,并且固有基团的存在限制了进一步转化的通用性。本文开发了一种羧酸促进的需氧催化系统,用于由简单的苯胺和酮一步合成吲哚。这种多功能的系统具有广泛的底物范围和使用环境氧气作为氧化剂的特点,对于实验室和工业应用均既方便又经济。C-3上不稳定的氢和C-2上高度可转化的羰基的存在,使吲哚在各种情况下成为有机合成的通用结构单元。基于密度泛函理论(DFT)的计算研究表明,决定速率的步骤是底物的羧酸辅助缩合,而不是芳基CH的官能化。因此,经由亚胺中间体的途径被认为是优选的机制。与一般的推论相反,据信原位形成的亚胺代替其烯胺异构体参与了α-Me的首次配体交换和随后的碳pal化,这为这种吲哚化机理提供了新的思路。































 京公网安备 11010802027423号
京公网安备 11010802027423号