当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stereoselective Synthesis of 3-Oxabicyclo[3.3.1]nonan-2-ones via a Domino Reaction Catalyzed by Modularly Designed Organocatalysts.
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2018-10-30 , DOI: 10.1002/adsc.201800987 Ramarao Parella 1 , Satish Jakkampudi 1 , Hadi Arman 1 , John C-G Zhao 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2018-10-30 , DOI: 10.1002/adsc.201800987 Ramarao Parella 1 , Satish Jakkampudi 1 , Hadi Arman 1 , John C-G Zhao 1
Affiliation
|
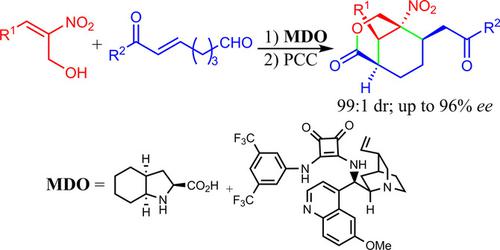
A highly stereoselective method for the synthesis of functionalized 3-oxabicyclo[3.3.1]nonan-2-one derivatives with four contiguous stereogenic centers, including one tetrasubstituted stereogenic center, was realized through an organocatalytic domino Michael-hemiacetalization-Michael reaction of (E)-3-aryl-2-nitroprop-2-enols and (E)-7-aryl-7-oxohept-5-enals followed by a PCC oxidation. Using the modularly designed organocatalysts (MDOs) self-assembled from cinchona alkaloid derivatives and amino acids in the reaction media, the title products were obtained in good yields (up to 84%), excellent diastereoselectivities (> 99:1 dr), and high enantioselectivities (up to 96% ee).
中文翻译:

通过模块化设计的有机催化剂催化的多米诺反应立体选择性合成 3-氧杂双环[3.3.1]壬南-2-酮。
通过(E )-3-芳基-2-硝基丙-2-烯醇和(E)-7-芳基-7-氧代庚基-5-烯醇,然后进行 PCC 氧化。使用由金鸡纳生物碱衍生物和氨基酸在反应介质中自组装的模块化设计的有机催化剂(MDO),以良好的收率(高达84%)和优异的非对映选择性(%3E 99:1 dr)获得了标题产物高对映选择性(高达 96% ee)。
更新日期:2018-11-26
中文翻译:

通过模块化设计的有机催化剂催化的多米诺反应立体选择性合成 3-氧杂双环[3.3.1]壬南-2-酮。
通过(E )-3-芳基-2-硝基丙-2-烯醇和(E)-7-芳基-7-氧代庚基-5-烯醇,然后进行 PCC 氧化。使用由金鸡纳生物碱衍生物和氨基酸在反应介质中自组装的模块化设计的有机催化剂(MDO),以良好的收率(高达84%)和优异的非对映选择性(%3E 99:1 dr)获得了标题产物高对映选择性(高达 96% ee)。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号