当前位置:
X-MOL 学术
›
Cell Death Differ.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Chlamydia trachomatis fails to protect its growth niche against pro-apoptotic insults.
Cell Death and Differentiation ( IF 13.7 ) Pub Date : 2018-Oct-30 , DOI: 10.1038/s41418-018-0224-2 Barbara S. Sixt , Carlos Núñez-Otero , Oliver Kepp , Raphael H. Valdivia , Guido Kroemer
Cell Death and Differentiation ( IF 13.7 ) Pub Date : 2018-Oct-30 , DOI: 10.1038/s41418-018-0224-2 Barbara S. Sixt , Carlos Núñez-Otero , Oliver Kepp , Raphael H. Valdivia , Guido Kroemer
|
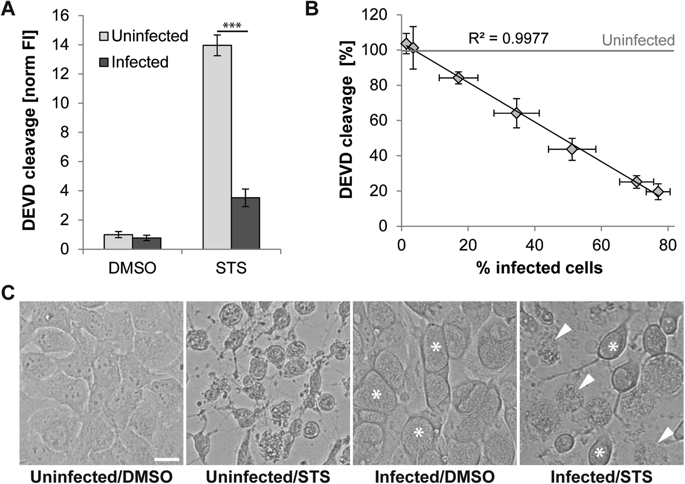
Chlamydia trachomatis is an obligate intracellular bacterial agent responsible for ocular infections and sexually transmitted diseases. It has been postulated that Chlamydia inhibits apoptosis in host cells to maintain an intact replicative niche until sufficient infectious progeny can be generated. Here we report that, while cells infected with C. trachomatis are protected from apoptosis at early and mid-stages of infection, they remain susceptible to the induction of other cell death modalities. By monitoring the fate of infected cells by time-lapse video microscopy and by analyzing host plasma membrane integrity and the activity of caspases, we determined that C. trachomatis-infected cells exposed to pro-apoptotic stimuli predominately died by a mechanism resembling necrosis. This necrotic death of infected cells occurred with kinetics similar to the induction of apoptosis in uninfected cells, indicating that C. trachomatis fails to considerably prolong the lifespan of its host cell when exposed to pro-apoptotic insults. Inhibitors of bacterial protein synthesis partially blocked necrotic death of infected cells, suggesting that the switch from apoptosis to necrosis relies on an active contribution of the bacteria. Tumor necrosis factor alpha (TNF-α)-mediated induction of necrosis in cells infected with C. trachomatis was not dependent on canonical regulators of necroptosis, such as RIPK1, RIPK3, or MLKL, yet was blocked by inhibition or depletion of CASP8. These results suggest that alternative signaling pathways regulate necrotic death in the context of C. trachomatis infections. Finally, consistent with the inability of C. trachomatis to preserve host cell viability, necrosis resulting from pro-apoptotic conditions significantly impaired production of infectious progeny. Taken together, our findings suggest that Chlamydia's anti-apoptotic activities are not sufficient to protect the pathogen's replicative niche.
中文翻译:

沙眼衣原体未能保护其生长小生境免受促细胞凋亡的侵害。
沙眼衣原体是专一的细胞内细菌剂,负责眼部感染和性传播疾病。据推测,衣原体抑制宿主细胞的凋亡以维持完整的复制位,直到可以产生足够的感染性子代。在这里我们报道,虽然感染了沙眼衣原体的细胞在感染的早期和中期受到保护免于凋亡,但它们仍然易于诱导其他细胞死亡方式。通过通过延时视频显微镜监测感染细胞的命运,并通过分析宿主的质膜完整性和胱天蛋白酶的活性,我们确定暴露于促凋亡刺激的沙眼衣原体感染的细胞主要是由类似于坏死的机制死亡。感染细胞的这种坏死以与未感染细胞中诱导凋亡相似的动力学发生,这表明沙眼衣原体在暴露于促凋亡的情况下不能显着延长其宿主细胞的寿命。细菌蛋白合成的抑制剂部分阻止了感染细胞的坏死性死亡,这表明从凋亡向坏死的转变依赖于细菌的积极贡献。在感染沙眼衣原体的细胞中,肿瘤坏死因子α(TNF-α)介导的坏死诱导不依赖于坏死病的规范性调节剂,例如RIPK1,RIPK3或MLKL,但被CASP8的抑制或消耗所阻断。这些结果表明,在沙眼衣原体感染的情况下,替代的信号传导途径调节了坏死性死亡。最后,与沙眼衣原体不能保存宿主细胞的活力相一致,促凋亡条件导致的坏死显着损害了感染后代的产生。综上所述,我们的发现表明衣原体的抗凋亡活性不足以保护病原体的复制生态位。
更新日期:2019-01-26
中文翻译:

沙眼衣原体未能保护其生长小生境免受促细胞凋亡的侵害。
沙眼衣原体是专一的细胞内细菌剂,负责眼部感染和性传播疾病。据推测,衣原体抑制宿主细胞的凋亡以维持完整的复制位,直到可以产生足够的感染性子代。在这里我们报道,虽然感染了沙眼衣原体的细胞在感染的早期和中期受到保护免于凋亡,但它们仍然易于诱导其他细胞死亡方式。通过通过延时视频显微镜监测感染细胞的命运,并通过分析宿主的质膜完整性和胱天蛋白酶的活性,我们确定暴露于促凋亡刺激的沙眼衣原体感染的细胞主要是由类似于坏死的机制死亡。感染细胞的这种坏死以与未感染细胞中诱导凋亡相似的动力学发生,这表明沙眼衣原体在暴露于促凋亡的情况下不能显着延长其宿主细胞的寿命。细菌蛋白合成的抑制剂部分阻止了感染细胞的坏死性死亡,这表明从凋亡向坏死的转变依赖于细菌的积极贡献。在感染沙眼衣原体的细胞中,肿瘤坏死因子α(TNF-α)介导的坏死诱导不依赖于坏死病的规范性调节剂,例如RIPK1,RIPK3或MLKL,但被CASP8的抑制或消耗所阻断。这些结果表明,在沙眼衣原体感染的情况下,替代的信号传导途径调节了坏死性死亡。最后,与沙眼衣原体不能保存宿主细胞的活力相一致,促凋亡条件导致的坏死显着损害了感染后代的产生。综上所述,我们的发现表明衣原体的抗凋亡活性不足以保护病原体的复制生态位。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号