Nano Energy ( IF 16.8 ) Pub Date : 2018-10-28 , DOI: 10.1016/j.nanoen.2018.10.040 Gebrekidan Gebresilassie Eshetu , Thomas Diemant , Maral Hekmatfar , Sylvie Grugeon , R. Jürgen Behm , Stephane Laruelle , Michel Armand , Stefano Passerini
|
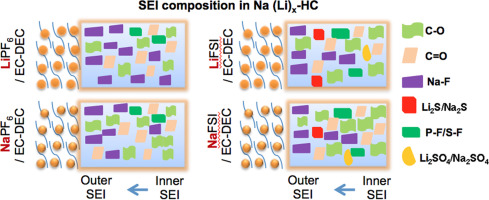
Aiming at a more comprehensive understanding of the solid electrolyte interphase (SEI) in sodium ion batteries (NIBs), a detailed X-ray photoelectron spectroscopy (XPS) investigation of the few-nanometer thick passivation film formed on hard carbon (HC) in contact with various Na+-ion conducting electrolytes is reported. The electrolytes investigated include 1 M solutions of NaPF6, NaClO4, NaTFSI, NaFSI, and NaFTFSI, all dissolved in a common mixture of ethylene carbonate (EC) and diethylene carbonate (DEC) (EC/DEC = 1/1 wt. ratio). For comparison, the study of analogous Li-based electrolytes containing LiPF6 and LiFSI as representative electrolyte salts is also reported. The anion and cation of the electrolyte salt appear to play a key role in determining the overall SEI layer composition, including its depth evolution and thickness. The SEI building species formed on hard carbon by solvent reduction upon sodiation are found to decrease with the various salts in the order: NaPF6 > NaClO4 ≈ NaTFSI > NaFTFSI > NaFSI. The comparison of lithiated and sodiated HC electrodes shows that the SEI layer is more homogeneous and richer in organic species upon the use of Na-based electrolytes. Surface and depth-profiling XPS analysis on HC electrodes charged in the various electrolyte formulations provides in-depth insights on the differences and similarities of the SEI (composition, thickness, depth evolution, etc.) evolving from the variation in the chemical structure of the cations and anions of the respective salts.
中文翻译:

电解质盐阴离子对钠离子电池中固体电解质界面形成的影响
为了更全面地了解钠离子电池(NIB)中的固体电解质相(SEI),详细研究了在接触的硬碳(HC)上形成的几纳米厚钝化膜的X射线光电子能谱(XPS)。据报道,具有各种Na +离子导电电解质。所研究的电解质包括1M NaPF 6,NaClO 4,NaTFSI,NaFSI和NaFTFSI溶液,所有溶液均溶解在碳酸亚乙酯(EC)和碳酸二亚乙酯(DEC)的普通混合物中(EC / DEC = 1/1重量比) )。为了进行比较,研究了含LiPF 6的类似锂基电解质还报道了LiFSI作为代表性的电解质盐。电解质盐的阴离子和阳离子似乎在确定整个SEI层组成(包括其深度演变和厚度)中起关键作用。已发现,通过腌制过程中溶剂还原后在硬碳上形成的SEI建筑物种会随着以下各种盐类的减少而减少:NaPF 6 > NaClO 4≈NaTFSI> NaFTFSI> NaFSI。锂和碳氢化合物HC电极的比较表明,在使用Na基电解质时,SEI层更均一且有机物更丰富。各种电解液配方中带电的HC电极的表面和深度剖析XPS分析提供了关于SEI的差异和相似性(成分,厚度,深度演变等)的深入见解,这些差异是由SEI的化学结构变化演变而来的各自盐的阳离子和阴离子。

































 京公网安备 11010802027423号
京公网安备 11010802027423号