当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantioselective and Regioselective Hydroetherification of Alkynes by Gold-Catalyzed Desymmetrization of Prochiral Phenols with P-Stereogenic Centers
Organic Letters ( IF 4.9 ) Pub Date : 2018-10-29 00:00:00 , DOI: 10.1021/acs.orglett.8b02982 Yin Zheng 1 , Linna Guo 1 , Weiwei Zi 1
Organic Letters ( IF 4.9 ) Pub Date : 2018-10-29 00:00:00 , DOI: 10.1021/acs.orglett.8b02982 Yin Zheng 1 , Linna Guo 1 , Weiwei Zi 1
Affiliation
|
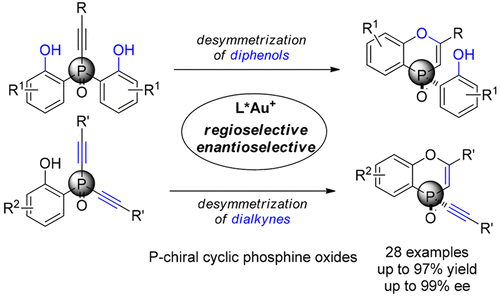
The gold(I)-catalyzed enantioselective hydroetherification of alkynes was achieved via desymmetrization of prochiral bisphenols bearing P-stereogenic centers. (S)-DTBM-Segphos(AuCl)2/AgNTf2 proved to be a highly efficient catalyst system for this transformation, affording P-chiral cyclic phosphine oxides in good yields with high enantioselectivities (with up to 99% ee). The same catalyst system allowed for the enantioselective desymmetrization of dialkynes. Synthetic transformations of the cyclization products afforded other P-chiral molecules with high enantiospecificity.
中文翻译:

金催化前体手性酚的不对称化与对映异构中心对炔烃的对映选择性和区域选择性加氢醚化
炔烃的金(I)催化的对映选择性水醚化是通过对带有P-立体异构中心的前手性双酚进行不对称化来实现的。(S)-DTBM-Segphos(AuCl)2 / AgNTf 2被证明是该转化的高效催化剂体系,以高收率和高对映选择性(高达99%ee)提供了P-手性环状氧化膦。相同的催化剂体系允许二酮的对映选择性脱对称。环化产物的合成转化提供了具有高对映体特异性的其他P-手性分子。
更新日期:2018-10-29
中文翻译:

金催化前体手性酚的不对称化与对映异构中心对炔烃的对映选择性和区域选择性加氢醚化
炔烃的金(I)催化的对映选择性水醚化是通过对带有P-立体异构中心的前手性双酚进行不对称化来实现的。(S)-DTBM-Segphos(AuCl)2 / AgNTf 2被证明是该转化的高效催化剂体系,以高收率和高对映选择性(高达99%ee)提供了P-手性环状氧化膦。相同的催化剂体系允许二酮的对映选择性脱对称。环化产物的合成转化提供了具有高对映体特异性的其他P-手性分子。































 京公网安备 11010802027423号
京公网安备 11010802027423号