当前位置:
X-MOL 学术
›
ACS Chem. Neurosci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
How Does Agonist and Antagonist Binding Lead to Different Conformational Ensemble Equilibria of the κ-Opioid Receptor: Insight from Long-Time Gaussian Accelerated Molecular Dynamics Simulation
ACS Chemical Neuroscience ( IF 4.1 ) Pub Date : 2018-10-29 00:00:00 , DOI: 10.1021/acschemneuro.8b00535 Xiaoli An 1 , Qifeng Bai 2 , Zhitong Bing 2, 3 , Shuangyan Zhou 1, 4 , Danfeng Shi 1 , Huanxiang Liu 4 , Xiaojun Yao 1, 5
ACS Chemical Neuroscience ( IF 4.1 ) Pub Date : 2018-10-29 00:00:00 , DOI: 10.1021/acschemneuro.8b00535 Xiaoli An 1 , Qifeng Bai 2 , Zhitong Bing 2, 3 , Shuangyan Zhou 1, 4 , Danfeng Shi 1 , Huanxiang Liu 4 , Xiaojun Yao 1, 5
Affiliation
|
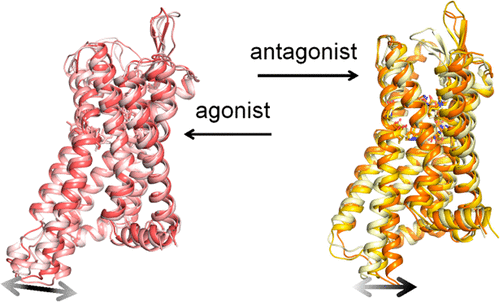
The opioid receptors belong to the class A seven transmembrane-spanning (7TM) G protein-coupled receptors (GPCRs). The κ-opioid receptor (KOR) is a subfamily of four opioid receptors. The endogenous peptide and a variety of selective agonists and antagonists of KOR have been developed. The structurally similar ligands at the same site cause completely opposite biological functions and induce different conformational changes. To shed light on the conformation ensembles and conformational dynamics in activation and deactivation processes of KOR, we performed all-atom, long-time Gaussian accelerated molecular dynamics simulation (GaMD) on KOR binding with agonist epoxymorphinan MP1104 and antagonist JDTic, respectively. Our results revealed different conformation ensembles of KOR binding with agonist and with antagonist. Agonist binding stabilizes the active state of key motifs including DYYNM motif and CWxP motif, and biases the conformation equilibria toward the active state. Antagonist binding will not destroy inactive conformation equilibria, by keeping the stable inactive state of these crucial motifs. We found that the inactive apo form of KOR is the most stable state, while the active apo form relaxes readily to inactive state. Our results also revealed a stable intermediate (I), which is attributed to the hydrophobic interactions between Tyr2465.58 and TM6, as well as the steric hindrance of them. Our results not only show the conformation equilibria bias of KOR by binding with agonist and antagonist, but also provide the structural information for the design and discovery of potential ligands with different functions.
中文翻译:

激动剂和拮抗剂的结合如何导致κ阿片受体的不同构象集合平衡:长期高斯加速分子动力学模拟的见解
阿片受体属于七类跨膜(7TM)G蛋白偶联受体(GPCR)。κ阿片受体(KOR)是四个阿片受体的一个亚家族。已经开发出内源肽和各种KOR选择性激动剂和拮抗剂。同一位点的结构相似的配体会导致完全相反的生物学功能,并诱导不同的构象变化。为了阐明KOR激活和失活过程中的构象集合和构象动力学,我们分别对激动剂环氧吗啡喃MP1104和拮抗剂JDTic进行了KOR结合的全原子,长时间高斯加速分子动力学模拟(GaMD)。我们的结果揭示了与激动剂和拮抗剂结合的KOR的不同构象集合。激动剂结合稳定了包括DYYNM基序和CWxP基序在内的关键基序的活跃状态,并使构象平衡偏向活跃状态。拮抗剂结合将通过保持这些关键基序的稳定非活性状态而不会破坏非活性构象平衡。我们发现,KOR的非活性载脂蛋白形式是最稳定的状态,而活性的载脂蛋白形式则容易松弛到非活性状态。我们的结果还揭示了稳定的中间体(I),这归因于Tyr246之间的疏水相互作用 我们发现,KOR的非活性载脂蛋白形式是最稳定的状态,而活性的载脂蛋白形式则容易松弛到非活性状态。我们的结果还揭示了稳定的中间体(I),这归因于Tyr246之间的疏水相互作用 我们发现,KOR的非活性载脂蛋白形式是最稳定的状态,而活性的载脂蛋白形式则容易松弛到非活性状态。我们的结果还揭示了稳定的中间体(I),这归因于Tyr246之间的疏水相互作用5.58和TM6,以及它们的空间位阻。我们的结果不仅显示了通过与激动剂和拮抗剂结合而形成的KOR的构象平衡偏差,而且还为设计和发现具有不同功能的潜在配体提供了结构信息。
更新日期:2018-10-29
中文翻译:

激动剂和拮抗剂的结合如何导致κ阿片受体的不同构象集合平衡:长期高斯加速分子动力学模拟的见解
阿片受体属于七类跨膜(7TM)G蛋白偶联受体(GPCR)。κ阿片受体(KOR)是四个阿片受体的一个亚家族。已经开发出内源肽和各种KOR选择性激动剂和拮抗剂。同一位点的结构相似的配体会导致完全相反的生物学功能,并诱导不同的构象变化。为了阐明KOR激活和失活过程中的构象集合和构象动力学,我们分别对激动剂环氧吗啡喃MP1104和拮抗剂JDTic进行了KOR结合的全原子,长时间高斯加速分子动力学模拟(GaMD)。我们的结果揭示了与激动剂和拮抗剂结合的KOR的不同构象集合。激动剂结合稳定了包括DYYNM基序和CWxP基序在内的关键基序的活跃状态,并使构象平衡偏向活跃状态。拮抗剂结合将通过保持这些关键基序的稳定非活性状态而不会破坏非活性构象平衡。我们发现,KOR的非活性载脂蛋白形式是最稳定的状态,而活性的载脂蛋白形式则容易松弛到非活性状态。我们的结果还揭示了稳定的中间体(I),这归因于Tyr246之间的疏水相互作用 我们发现,KOR的非活性载脂蛋白形式是最稳定的状态,而活性的载脂蛋白形式则容易松弛到非活性状态。我们的结果还揭示了稳定的中间体(I),这归因于Tyr246之间的疏水相互作用 我们发现,KOR的非活性载脂蛋白形式是最稳定的状态,而活性的载脂蛋白形式则容易松弛到非活性状态。我们的结果还揭示了稳定的中间体(I),这归因于Tyr246之间的疏水相互作用5.58和TM6,以及它们的空间位阻。我们的结果不仅显示了通过与激动剂和拮抗剂结合而形成的KOR的构象平衡偏差,而且还为设计和发现具有不同功能的潜在配体提供了结构信息。















































 京公网安备 11010802027423号
京公网安备 11010802027423号