当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Selective Capture of Phenol from Biofuel Using Protonated Faujasite Zeolites with Different Si/Al Ratios
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2018-11-08 , DOI: 10.1021/acs.jpcc.8b07875 Ibrahim Khalil 1 , Hicham Jabraoui 2 , Guillaume Maurin 3 , Sébastien Lebègue 2 , Michael Badawi 2 , Karine Thomas 1 , Francoise Maugé 1
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2018-11-08 , DOI: 10.1021/acs.jpcc.8b07875 Ibrahim Khalil 1 , Hicham Jabraoui 2 , Guillaume Maurin 3 , Sébastien Lebègue 2 , Michael Badawi 2 , Karine Thomas 1 , Francoise Maugé 1
Affiliation
|
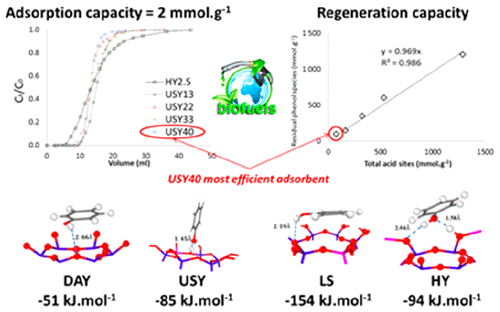
The purification of biofuels becomes a challenging issue because of the harmfulness of remaining phenolic molecules for human health and engines. To this end, protonic Y zeolites with different Si/Al ratios were explored as effective adsorbent materials to remove phenol from isooctane solution by using a dual experimental/computational strategy. Phenol was selectively removed from isooctane over HY and USY zeolites with a maximal adsorption capacity of 2.2 mmol·g–1, which corresponds to 3–4 phenol molecules per zeolitic supercage. The adsorption equilibrium was reached faster over dealuminated zeolites, due to the presence of large pores at the expense of microporosity as well as a low density of acidic sites. We further evidence that the presence of acid sites limits the regeneration capacity since phenol was strongly adsorbed on both Brønsted and Lewis acid sites. USY zeolite with the highest Si/Al ratio presents the best regeneration capacity since it has the lower aluminum loading. A fundamental understanding of these performances was obtained by coupling characterization (infrared spectroscopy, breakthrough curves, and desorption experiments) and modeling tools (Grand Canonical Monte Carlo and Density Functional Theory).
中文翻译:

使用不同Si / Al比的质子化八面沸石从生物燃料中选择性捕获苯酚
由于残留酚类分子对人体健康和发动机的危害,生物燃料的纯化成为一个具有挑战性的问题。为此,通过使用双重实验/计算策略,探索了具有不同Si / Al比的质子型Y沸石作为从异辛烷溶液中去除苯酚的有效吸附材料。在HY和USY沸石上从异辛烷中选择性去除苯酚,最大吸附容量为2.2 mmol·g –1,每个沸石超笼子对应3-4个酚分子。由于存在大孔而以微孔性为代价以及酸性位点的密度较低,因此与脱铝沸石相比,可以更快地达到吸附平衡。我们进一步证明酸位的存在限制了再生能力,因为苯酚强烈吸附在布朗斯台德和路易斯酸位上。具有最高Si / Al比的USY沸石具有最佳的再生能力,因为它具有较低的铝负载量。对这些性能的基本了解是通过耦合表征(红外光谱,穿透曲线和解吸实验)和建模工具(大经典蒙特卡洛和密度泛函理论)获得的。
更新日期:2018-11-09
中文翻译:

使用不同Si / Al比的质子化八面沸石从生物燃料中选择性捕获苯酚
由于残留酚类分子对人体健康和发动机的危害,生物燃料的纯化成为一个具有挑战性的问题。为此,通过使用双重实验/计算策略,探索了具有不同Si / Al比的质子型Y沸石作为从异辛烷溶液中去除苯酚的有效吸附材料。在HY和USY沸石上从异辛烷中选择性去除苯酚,最大吸附容量为2.2 mmol·g –1,每个沸石超笼子对应3-4个酚分子。由于存在大孔而以微孔性为代价以及酸性位点的密度较低,因此与脱铝沸石相比,可以更快地达到吸附平衡。我们进一步证明酸位的存在限制了再生能力,因为苯酚强烈吸附在布朗斯台德和路易斯酸位上。具有最高Si / Al比的USY沸石具有最佳的再生能力,因为它具有较低的铝负载量。对这些性能的基本了解是通过耦合表征(红外光谱,穿透曲线和解吸实验)和建模工具(大经典蒙特卡洛和密度泛函理论)获得的。































 京公网安备 11010802027423号
京公网安备 11010802027423号