当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Photophysical and Protonation Time Resolved Studies of Donor–Acceptor Branched Systems With Pyridine Acceptors
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2018-10-26 00:00:00 , DOI: 10.1021/acs.jpca.8b08628 Fotis Kournoutas 1 , Kostas Seintis 1 , Nikolaos Karakostas 2 , Jiří Tydlitát 3 , Sylvain Achelle 4 , George Pistolis 2 , Filip Bureš 3 , Mihalis Fakis 1
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2018-10-26 00:00:00 , DOI: 10.1021/acs.jpca.8b08628 Fotis Kournoutas 1 , Kostas Seintis 1 , Nikolaos Karakostas 2 , Jiří Tydlitát 3 , Sylvain Achelle 4 , George Pistolis 2 , Filip Bureš 3 , Mihalis Fakis 1
Affiliation
|
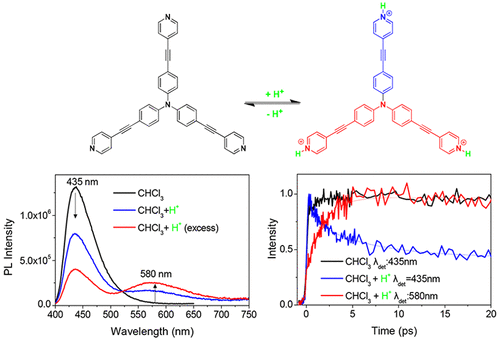
A comparative study of the photophysical properties of octupolar pyridyl-terminated triphenylamine molecule, with its quadrupolar and dipolar analogues, by means of ambient and low temperature steady state spectroscopy and femtosecond to nanosecond time-resolved fluorescence spectroscopy is reported. The push–pull molecules bear triphenylamine electron donating core, pyridine peripheral electron acceptors, and acetylene π-bridge. The samples were studied in solvents of varying polarity and also upon addition of small amounts of acetic acid to induce protonation of the pyridine group. All samples exhibit significant positive fluorescence solvatochromism as well as a relaxation of their excited state to a solvent relaxed intramolecular charge transfer state on the picosecond time scale. For the octupolar compound, excited state relaxation occurs simultaneously with excitation energy hopping among the branches. The hopping time is solvent polarity controlled since it becomes slower as the polarity increases. The experimental hopping times are compared to those predicted by Förster and Fermi formulations. The samples are capable of emitting broadband light covering almost the whole visible spectrum by careful control of protonation. Energy transfer from the neutral toward the protonated species on the 1 ps time scale is revealed.
中文翻译:

具有吡啶受体的供体-受体分支系统的光物理和质子化时间解析
通过环境和低温稳态光谱法和飞秒至纳秒时间分辨荧光光谱法,对八极吡啶基封端的三苯基胺分子及其四极和偶极类似物的光物理性质进行了比较研究。推挽分子带有三苯胺供电子核,吡啶外围电子受体和乙炔π桥。在不同极性的溶剂中以及在添加少量乙酸以诱导吡啶基的质子化之后,对样品进行了研究。所有样品均表现出显着的正荧光溶剂变色作用,并且在皮秒级的时间范围内,其激发态弛豫为溶剂弛豫的分子内电荷转移态。对于八极化合物,激发态弛豫与分支之间的激发能跳跃同时发生。跳跃时间受溶剂极性控制,因为随着极性的增加,跳跃时间变慢。将实验跳跃时间与Förster和Fermi公式预测的跳跃时间进行比较。通过仔细控制质子化,样品能够发射几乎覆盖整个可见光谱的宽带光。揭示了在1 ps的时间尺度上从中性物质向质子化物质的能量转移。通过仔细控制质子化,样品能够发射几乎覆盖整个可见光谱的宽带光。揭示了在1 ps的时间尺度上从中性物质向质子化物质的能量转移。通过仔细控制质子化,样品能够发射几乎覆盖整个可见光谱的宽带光。揭示了在1 ps的时间尺度上从中性物质向质子化物质的能量转移。
更新日期:2018-10-26
中文翻译:

具有吡啶受体的供体-受体分支系统的光物理和质子化时间解析
通过环境和低温稳态光谱法和飞秒至纳秒时间分辨荧光光谱法,对八极吡啶基封端的三苯基胺分子及其四极和偶极类似物的光物理性质进行了比较研究。推挽分子带有三苯胺供电子核,吡啶外围电子受体和乙炔π桥。在不同极性的溶剂中以及在添加少量乙酸以诱导吡啶基的质子化之后,对样品进行了研究。所有样品均表现出显着的正荧光溶剂变色作用,并且在皮秒级的时间范围内,其激发态弛豫为溶剂弛豫的分子内电荷转移态。对于八极化合物,激发态弛豫与分支之间的激发能跳跃同时发生。跳跃时间受溶剂极性控制,因为随着极性的增加,跳跃时间变慢。将实验跳跃时间与Förster和Fermi公式预测的跳跃时间进行比较。通过仔细控制质子化,样品能够发射几乎覆盖整个可见光谱的宽带光。揭示了在1 ps的时间尺度上从中性物质向质子化物质的能量转移。通过仔细控制质子化,样品能够发射几乎覆盖整个可见光谱的宽带光。揭示了在1 ps的时间尺度上从中性物质向质子化物质的能量转移。通过仔细控制质子化,样品能够发射几乎覆盖整个可见光谱的宽带光。揭示了在1 ps的时间尺度上从中性物质向质子化物质的能量转移。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号