当前位置:
X-MOL 学术
›
J. Ind. Eng. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Elucidation of Hydrolysis Reaction Mechanism of Tungsten Hexafluoride (WF6) Using First-Principles Calculations
Journal of Industrial and Engineering Chemistry ( IF 5.9 ) Pub Date : 2019-02-01 , DOI: 10.1016/j.jiec.2018.10.024 Hyunwook Jung , Jeemin Hwang , Hoje Chun , Byungchan Han
Journal of Industrial and Engineering Chemistry ( IF 5.9 ) Pub Date : 2019-02-01 , DOI: 10.1016/j.jiec.2018.10.024 Hyunwook Jung , Jeemin Hwang , Hoje Chun , Byungchan Han
|
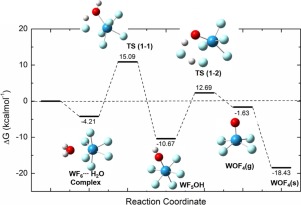
Abstract We identify hydrolysis reaction mechanism of water-reactive WF6 and its accompanying intermediates using first-principles calculations. For the purpose, we evaluate activation and free energy diagrams of elementary reaction steps. We find that WF6, WOF4, and WO2F2 form stable adducts, which quickly reacts with H2O by substituting the ligand F. Gaseous WOF4, WO2F2, WO3 are predicted as unstable in the increasing order, but polymerization reduces their instability, leading to solidification. In overall reaction, WOF4 hydrolysis is the bottleneck due to significantly higher activation barrier of trans isomeric complex than cis counterpart.
中文翻译:

使用第一性原理计算阐明六氟化钨 (WF6) 的水解反应机理
摘要 我们使用第一性原理计算确定了水反应性 WF6 及其伴随中间体的水解反应机理。为此,我们评估了基本反应步骤的活化和自由能图。我们发现 WF6、WOF4 和 WO2F2 形成稳定的加合物,通过取代配体 F 与 H2O 快速反应。预计气态 WOF4、WO2F2、WO3 按递增顺序不稳定,但聚合降低了它们的不稳定性,导致固化。在整个反应中,WOF4 水解是瓶颈,因为反式异构体复合物的活化屏障明显高于顺式对应物。
更新日期:2019-02-01
中文翻译:

使用第一性原理计算阐明六氟化钨 (WF6) 的水解反应机理
摘要 我们使用第一性原理计算确定了水反应性 WF6 及其伴随中间体的水解反应机理。为此,我们评估了基本反应步骤的活化和自由能图。我们发现 WF6、WOF4 和 WO2F2 形成稳定的加合物,通过取代配体 F 与 H2O 快速反应。预计气态 WOF4、WO2F2、WO3 按递增顺序不稳定,但聚合降低了它们的不稳定性,导致固化。在整个反应中,WOF4 水解是瓶颈,因为反式异构体复合物的活化屏障明显高于顺式对应物。































 京公网安备 11010802027423号
京公网安备 11010802027423号