当前位置:
X-MOL 学术
›
Tetrahedron
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
New efficient synthesis of 1H-imidazo-[4,5-c]quinolines by a sequential Van Leusen/Staudinger/aza-Wittig/carbodiimide-mediated cyclization
Tetrahedron ( IF 2.1 ) Pub Date : 2018-10-25 , DOI: 10.1016/j.tet.2018.10.052 Zhi-Rong Guan , Zi-Ming Liu , Ming-Wu Ding
中文翻译:

连续Van Leusen / Staudinger / aza-Wittig / carbodiimide介导的环化反应新合成1 H-咪唑并[4,5- c ]喹啉
更新日期:2018-10-25
Tetrahedron ( IF 2.1 ) Pub Date : 2018-10-25 , DOI: 10.1016/j.tet.2018.10.052 Zhi-Rong Guan , Zi-Ming Liu , Ming-Wu Ding
|
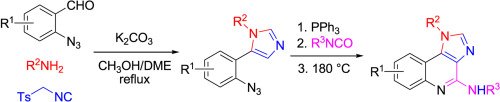
A new efficient synthesis of multisubstituted 1H-imidazo-[4,5-c]quinoline derivatives via sequential van Leusen/Staudinger/aza-Wittig/carbodiimide-mediated cyclization was developed. Azides 4, obtained from Van Leusen reaction of 2-azidobenzaldehyde 1, amine 2 and TosMIC (tosylmethyl isocyanide) 3, reacted with triphenyl phosphine to produce iminophosphorane 5. A tandem aza-Wittig reaction of iminophosphorane 5 with isocyanate generated 1H-imidazo-[4,5-c]quinoline 7 through carbodiimide intermediate 6 in moderate to good yield.
中文翻译:

连续Van Leusen / Staudinger / aza-Wittig / carbodiimide介导的环化反应新合成1 H-咪唑并[4,5- c ]喹啉
通过顺序范勒森/施陶丁格/氮杂-维蒂希/碳二亚胺介导的环化反应,开发了一种新的高效合成多取代的1 H-咪唑基-[4,5- c ]喹啉衍生物的方法。从2-叠氮苯甲醛1,胺2和TosMIC(甲苯磺酰基甲基异氰化物)3的Van Leusen反应中获得的叠氮化物4与三苯基膦反应生成亚氨基膦5。亚氨基磷烷5与异氰酸酯的串联氮杂-维蒂希反应通过碳二亚胺中间体6产生1 H-咪唑基-[4,5- c ]喹啉7,产率中等至良好。















































 京公网安备 11010802027423号
京公网安备 11010802027423号