当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mass Transport Analysis of the Enhanced Buffer Capacity of the Bicarbonate–CO2 Buffer in a Phase-Heterogenous System: Physiological and Pharmaceutical Significance
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2018-10-26 00:00:00 , DOI: 10.1021/acs.molpharmaceut.8b00783 Jozef Al-Gousous 1 , Kathy X. Sun 1 , Daniel P. McNamara 2 , Bart Hens 1 , Niloufar Salehi 1 , Peter Langguth 3 , Marival Bermejo 4 , Gregory E. Amidon 1 , Gordon L. Amidon 1
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2018-10-26 00:00:00 , DOI: 10.1021/acs.molpharmaceut.8b00783 Jozef Al-Gousous 1 , Kathy X. Sun 1 , Daniel P. McNamara 2 , Bart Hens 1 , Niloufar Salehi 1 , Peter Langguth 3 , Marival Bermejo 4 , Gregory E. Amidon 1 , Gordon L. Amidon 1
Affiliation
|
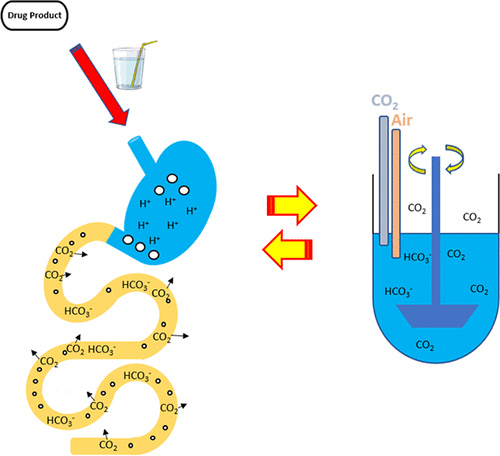
The bicarbonate buffer capacity is usually considered in a phase-homogeneous system, at equilibrium, with no CO2 transfer between the liquid buffer phase and another phase. However, typically, an in vitro bicarbonate buffer-based system is a phase-heterogeneous system, as it entails continuously sparging (bubbling) the dissolution medium with CO2 in a gas mixture, at constant ratio, to maintain a constant partial pressure of CO2 (g) and CO2(aq) molarity at a prescribed value, with CO2 diffusing freely between the gas and the aqueous phases. The human gastrointestinal tract is also a phase-heterogeneous system, with CO2 diffusing across the mucosal membrane into the mesenteric arterial blood, which serves as a sink for CO2 from the intestinal lumen. In this report, a mass transport analysis of the apparent buffer capacity of a phase-heterogeneous bicarbonate–CO2 system is developed. It is shown that, most significantly, a phase-heterogeneous bicarbonate–CO2 system can have a much higher buffer capacity than a phase-homogeneous system such that the buffer capacity is dependent on the bicarbonate concentration. It is double that of a phase-homogeneous system at the pH = pKa for a monoprotic buffer at the same concentration. This buffer capacity enhancement increases hyperbolically with pH above the pKa, thus providing a much stronger buffering to keep the pH in the physiologically neutral range. The buffer capacity will be dependent on the bicarbonate molarity (which in vivo will depend on the bicarbonate secretion rate) and not the pH of the luminal fluid. Further, there is no conjugate acid accumulation as a result of bicarbonate neutralization, since the resulting carbonic acid (H2CO3) rapidly dehydrates producing CO2 and H2O. The mass transport analysis developed in this report is further supported by in vitro experimental results. This enhanced bicarbonate buffer capacity in a phase-heterogeneous system is of physiological significance as well as significant for the dissolution and absorption of ionizable drugs.
中文翻译:

相异质系统中碳酸氢盐-CO 2缓冲液增强的缓冲容量的传质分析:生理和药学意义
通常认为碳酸氢盐的缓冲能力是在均相的系统中,处于平衡状态,液体缓冲相和另一相之间没有CO 2转移。但是,通常,基于体外碳酸氢盐缓冲液的系统是相异相系统,因为它需要在混合气体中以恒定比率连续向鼓泡(鼓泡)溶解介质和CO 2,以保持恒定的CO分压。2(g)和CO 2(aq)摩尔浓度处于规定值,CO 2在气相和水相之间自由扩散。人的胃肠道也是异相系统,具有CO 2穿过粘膜扩散进入肠系膜动脉血液,该血液用作肠腔中CO 2的汇。在本报告中,对相异质碳酸氢盐-CO 2系统的表观缓冲容量进行了传质分析。结果表明,最重要的是,相均相的碳酸氢盐-CO 2系统比相均相的系统具有更高的缓冲容量,因此缓冲容量取决于碳酸氢盐的浓度。对于相同浓度的单质子缓冲液,在pH = p K a时,它是相均相系统的两倍。当pH值高于p K a时,这种缓冲能力的增强将大大增加。,因此提供了更强的缓冲作用,可将pH值保持在生理中性范围内。缓冲能力将取决于碳酸氢盐的摩尔浓度(其在体内将取决于碳酸氢盐的分泌速率),而不取决于腔液的pH。此外,不存在共轭酸的积累作为碳酸氢盐中和的结果,因为得到的碳酸(H 2 CO 3)迅速脱水产生CO 2和H 2 O.在本报告中所开发的质量运输分析是通过进一步支持体外实验结果。相不均相系统中碳酸氢盐缓冲液容量的增加,不仅具有生理意义,而且对可离子化药物的溶解和吸收也具有重要意义。
更新日期:2018-10-26
中文翻译:

相异质系统中碳酸氢盐-CO 2缓冲液增强的缓冲容量的传质分析:生理和药学意义
通常认为碳酸氢盐的缓冲能力是在均相的系统中,处于平衡状态,液体缓冲相和另一相之间没有CO 2转移。但是,通常,基于体外碳酸氢盐缓冲液的系统是相异相系统,因为它需要在混合气体中以恒定比率连续向鼓泡(鼓泡)溶解介质和CO 2,以保持恒定的CO分压。2(g)和CO 2(aq)摩尔浓度处于规定值,CO 2在气相和水相之间自由扩散。人的胃肠道也是异相系统,具有CO 2穿过粘膜扩散进入肠系膜动脉血液,该血液用作肠腔中CO 2的汇。在本报告中,对相异质碳酸氢盐-CO 2系统的表观缓冲容量进行了传质分析。结果表明,最重要的是,相均相的碳酸氢盐-CO 2系统比相均相的系统具有更高的缓冲容量,因此缓冲容量取决于碳酸氢盐的浓度。对于相同浓度的单质子缓冲液,在pH = p K a时,它是相均相系统的两倍。当pH值高于p K a时,这种缓冲能力的增强将大大增加。,因此提供了更强的缓冲作用,可将pH值保持在生理中性范围内。缓冲能力将取决于碳酸氢盐的摩尔浓度(其在体内将取决于碳酸氢盐的分泌速率),而不取决于腔液的pH。此外,不存在共轭酸的积累作为碳酸氢盐中和的结果,因为得到的碳酸(H 2 CO 3)迅速脱水产生CO 2和H 2 O.在本报告中所开发的质量运输分析是通过进一步支持体外实验结果。相不均相系统中碳酸氢盐缓冲液容量的增加,不仅具有生理意义,而且对可离子化药物的溶解和吸收也具有重要意义。















































 京公网安备 11010802027423号
京公网安备 11010802027423号