当前位置:
X-MOL 学术
›
Nat. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Non-catalytic signaling by pseudokinase ILK for regulating cell adhesion.
Nature Communications ( IF 14.7 ) Pub Date : 2018-10-26 , DOI: 10.1038/s41467-018-06906-7 Julia Vaynberg 1 , Koichi Fukuda 1 , Fan Lu 1, 2 , Katarzyna Bialkowska 1 , Yinghua Chen 3 , Edward F Plow 1 , Jun Qin 1, 2
Nature Communications ( IF 14.7 ) Pub Date : 2018-10-26 , DOI: 10.1038/s41467-018-06906-7 Julia Vaynberg 1 , Koichi Fukuda 1 , Fan Lu 1, 2 , Katarzyna Bialkowska 1 , Yinghua Chen 3 , Edward F Plow 1 , Jun Qin 1, 2
Affiliation
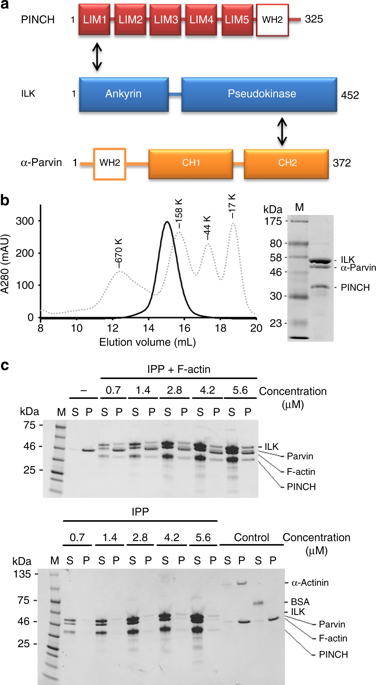
|
Dynamic communication between integrin-containing complexes (focal adhesions, FAs) and actin filaments is critical for regulating cell adhesion. Pseudokinase ILK plays a key role in this process but the underlying mechanism remains highly elusive. Here we show that by recruiting FA adaptors PINCH and Parvin into a heterotrimeric complex (IPP), ILK triggers F-actin filament bundling - a process known to generate force/mechanical signal to promote cytoskeleton reassembly and dynamic cell adhesion. Structural, biochemical, and functional analyses revealed that the F-actin bundling is orchestrated by two previously unrecognized WASP-Homology-2 actin binding motifs within IPP, one from PINCH and the other from Parvin. Strikingly, this process is also sensitized to Mg-ATP bound to the pseudoactive site of ILK and its dysregulation severely impairs stress fibers formation, cell spreading, and migration. These data identify a crucial mechanism for ILK, highlighting its uniqueness as a pseudokinase to transduce non-catalytic signal and regulate cell adhesion.
中文翻译:

假激酶 ILK 的非催化信号传导用于调节细胞粘附。
含整合素的复合物(粘着斑,FA)和肌动蛋白丝之间的动态通讯对于调节细胞粘附至关重要。假激酶 ILK 在此过程中发挥着关键作用,但其潜在机制仍然非常难以捉摸。在这里,我们展示了通过将 FA 接头 PINCH 和 Parvin 募集到异源三聚体复合物 (IPP) 中,ILK 触发 F-肌动蛋白丝成束,这是一个已知会产生力/机械信号以促进细胞骨架重新组装和动态细胞粘附的过程。结构、生化和功能分析表明,F-肌动蛋白捆绑是由 IPP 内两个先前未被识别的 WASP-Homology-2 肌动蛋白结合基序精心策划的,一个来自 PINCH,另一个来自 Parvin。引人注目的是,该过程还对与 ILK 假活性位点结合的 Mg-ATP 敏感,其失调会严重损害应力纤维的形成、细胞扩散和迁移。这些数据确定了 ILK 的关键机制,突出了其作为假激酶转导非催化信号和调节细胞粘附的独特性。
更新日期:2018-10-26
中文翻译:

假激酶 ILK 的非催化信号传导用于调节细胞粘附。
含整合素的复合物(粘着斑,FA)和肌动蛋白丝之间的动态通讯对于调节细胞粘附至关重要。假激酶 ILK 在此过程中发挥着关键作用,但其潜在机制仍然非常难以捉摸。在这里,我们展示了通过将 FA 接头 PINCH 和 Parvin 募集到异源三聚体复合物 (IPP) 中,ILK 触发 F-肌动蛋白丝成束,这是一个已知会产生力/机械信号以促进细胞骨架重新组装和动态细胞粘附的过程。结构、生化和功能分析表明,F-肌动蛋白捆绑是由 IPP 内两个先前未被识别的 WASP-Homology-2 肌动蛋白结合基序精心策划的,一个来自 PINCH,另一个来自 Parvin。引人注目的是,该过程还对与 ILK 假活性位点结合的 Mg-ATP 敏感,其失调会严重损害应力纤维的形成、细胞扩散和迁移。这些数据确定了 ILK 的关键机制,突出了其作为假激酶转导非催化信号和调节细胞粘附的独特性。

































 京公网安备 11010802027423号
京公网安备 11010802027423号