当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of Furan-Annelated BINOL Derivatives: Acid-Catalyzed Cyclization Induces Partial Racemization
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2018-10-25 00:00:00 , DOI: 10.1021/acs.joc.8b02353 Frescilia Octa-Smolin 1 , Felix van der Vight 2 , Rohan Yadav 1 , Jasmine Bhangu 1 , Kateryna Soloviova 3 , Christoph Wölper 4 , Constantin G. Daniliuc 5 , Cristian A. Strassert 3 , Holger Somnitz 2 , Georg Jansen 2 , Jochen Niemeyer 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2018-10-25 00:00:00 , DOI: 10.1021/acs.joc.8b02353 Frescilia Octa-Smolin 1 , Felix van der Vight 2 , Rohan Yadav 1 , Jasmine Bhangu 1 , Kateryna Soloviova 3 , Christoph Wölper 4 , Constantin G. Daniliuc 5 , Cristian A. Strassert 3 , Holger Somnitz 2 , Georg Jansen 2 , Jochen Niemeyer 1
Affiliation
|
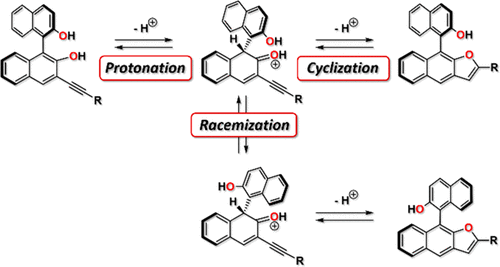
In this account, we describe the synthesis of a series of BINOL-based bis- and trisphosphoric acids 11d/e/f, which commonly feature an unusual phosphoric acid monoester motif. This motif is generated by an acid-catalyzed 5-endo-dig cyclization of the 3-alkynyl-substituted BINOL precursors to give the corresponding Furan-annelated derivatives, followed by phosphorylation of the remaining phenolic alcohols. In the cyclization reaction, we observed an unexpected partial racemization in the bis- and tris-BINOL scaffolds, leading to mixtures of diastereomers that were separated and characterized spectroscopically and by X-ray crystal structure analyses. The cyclization and racemization processes were investigated both experimentally and by DFT-calculations, showing that although the cyclization proceeds faster, the barrier for the acid-catalyzed binaphthyl-racemization is only slightly higher.
中文翻译:

呋喃环化BINOL衍生物的合成:酸催化的环化诱导部分消旋
在这个帐户中,我们描述了一系列基于BINOL的双磷酸和三磷酸11d / e / f的合成,它们通常具有不寻常的磷酸单酯基序。该基序是由酸催化的5-产生内切-挖将3-炔基取代的BINOL前体环化,得到相应的呋喃-酰胺化衍生物,然后将剩余的酚醇磷酸化。在环化反应中,我们在bis-和tris-BINOL支架中观察到了意外的部分外消旋作用,导致了非对映体混合物的分离,并通过光谱和X射线晶体结构分析对其进行了表征。通过实验和DFT计算研究了环化和外消旋化过程,结果表明,尽管环化进行得更快,但酸催化的联萘外消旋的壁垒仅稍高一些。
更新日期:2018-10-25
中文翻译:

呋喃环化BINOL衍生物的合成:酸催化的环化诱导部分消旋
在这个帐户中,我们描述了一系列基于BINOL的双磷酸和三磷酸11d / e / f的合成,它们通常具有不寻常的磷酸单酯基序。该基序是由酸催化的5-产生内切-挖将3-炔基取代的BINOL前体环化,得到相应的呋喃-酰胺化衍生物,然后将剩余的酚醇磷酸化。在环化反应中,我们在bis-和tris-BINOL支架中观察到了意外的部分外消旋作用,导致了非对映体混合物的分离,并通过光谱和X射线晶体结构分析对其进行了表征。通过实验和DFT计算研究了环化和外消旋化过程,结果表明,尽管环化进行得更快,但酸催化的联萘外消旋的壁垒仅稍高一些。































 京公网安备 11010802027423号
京公网安备 11010802027423号