当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nanometric water channels in water-in-salt lithium-ion battery electrolyte
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-10-25 , DOI: 10.1021/jacs.8b07696 Joonhyung Lim 1, 2 , Kwanghee Park 1, 2 , Hochan Lee 1, 2 , Jungyu Kim 1, 2 , Kyungwon Kwak 1, 2 , Minhaeng Cho 1, 2
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-10-25 , DOI: 10.1021/jacs.8b07696 Joonhyung Lim 1, 2 , Kwanghee Park 1, 2 , Hochan Lee 1, 2 , Jungyu Kim 1, 2 , Kyungwon Kwak 1, 2 , Minhaeng Cho 1, 2
Affiliation
|
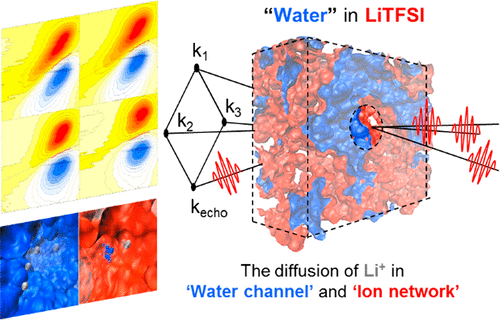
Lithium-ion batteries (LIBs) have been deployed in a wide range of energy-storage applications and helped to revolutionize technological development. Recently, a lithium ion battery that uses superconcentrated salt water as its electrolyte has been developed. However, the role of water in facilitating fast ion transport in such highly concentrated electrolyte solutions is not fully understood yet. Here, femtosecond IR spectroscopy and molecular dynamics simulations are used to show that bulk-like water coexists with interfacial water on ion aggregates. We found that dissolved ions form intricate three-dimensional ion-ion networks that are spontaneously intertwined with nanometric water hydrogen-bonding networks. Then, hydrated lithium ions move through bulk-like water channels acting like conducting wires for lithium ion transport. Our experimental and simulation results indicate that water structure-breaking chaotropic anion salts with a high propensity to form ion networks in aqueous solutions would be excellent candidates for water-based LIB electrolytes. We anticipate that the present work will provide guiding principles for developing aqueous LIB electrolytes.
中文翻译:

盐包水锂离子电池电解液中的纳米水通道
锂离子电池 (LIB) 已部署在广泛的储能应用中,并有助于彻底改变技术发展。最近,已经开发出使用超浓盐水作为电解质的锂离子电池。然而,水在促进这种高浓度电解质溶液中快速离子传输方面的作用尚不完全清楚。在这里,飞秒 IR 光谱和分子动力学模拟被用来表明块状水与离子聚集体上的界面水共存。我们发现溶解的离子形成复杂的三维离子-离子网络,这些网络与纳米水氢键网络自发地交织在一起。然后,水合锂离子穿过大块状的水通道,其作用就像用于锂离子传输的导线。我们的实验和模拟结果表明,在水溶液中具有形成离子网络的高倾向的破坏水结构的离液阴离子盐将是水基 LIB 电解质的极好候选者。我们预计目前的工作将为开发水性 LIB 电解质提供指导原则。
更新日期:2018-10-25
中文翻译:

盐包水锂离子电池电解液中的纳米水通道
锂离子电池 (LIB) 已部署在广泛的储能应用中,并有助于彻底改变技术发展。最近,已经开发出使用超浓盐水作为电解质的锂离子电池。然而,水在促进这种高浓度电解质溶液中快速离子传输方面的作用尚不完全清楚。在这里,飞秒 IR 光谱和分子动力学模拟被用来表明块状水与离子聚集体上的界面水共存。我们发现溶解的离子形成复杂的三维离子-离子网络,这些网络与纳米水氢键网络自发地交织在一起。然后,水合锂离子穿过大块状的水通道,其作用就像用于锂离子传输的导线。我们的实验和模拟结果表明,在水溶液中具有形成离子网络的高倾向的破坏水结构的离液阴离子盐将是水基 LIB 电解质的极好候选者。我们预计目前的工作将为开发水性 LIB 电解质提供指导原则。































 京公网安备 11010802027423号
京公网安备 11010802027423号