当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Solvent and Salt Effect on Lithium Ion Solvation and Contact Ion Pair Formation in Organic Carbonates: A Quantum Chemical Perspective
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2018-11-07 , DOI: 10.1021/acs.jpcc.8b09892 Veerapandian Ponnuchamy 1, 2 , Stefano Mossa 1 , Ioannis Skarmoutsos 1
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2018-11-07 , DOI: 10.1021/acs.jpcc.8b09892 Veerapandian Ponnuchamy 1, 2 , Stefano Mossa 1 , Ioannis Skarmoutsos 1
Affiliation
|
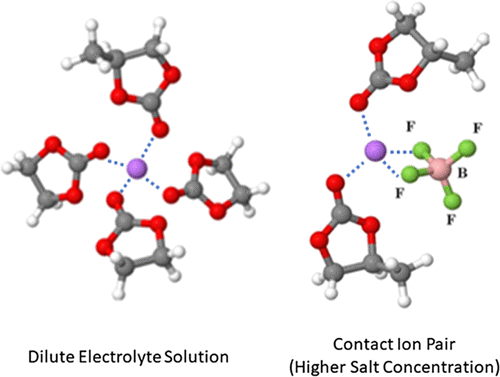
Quantum chemical calculations have been employed to investigate the solvation of lithium cations in ethylene carbonate/propylene carbonate and propylene carbonate/dimethyl carbonate mixed electrolytes. The impact of the presence of the counteranion on the solvation of Li+ in pure propylene carbonate and dimethyl carbonate was also studied. The calculations revealed small free-energy changes for the transitions between different preferred structures in mixed solvents. This implies that transitions between distinct local arrangements can take place in the mixtures. The addition of dimethyl carbonate causes a significant increase of the dipole moment of solvation clusters, indicating important molecular-scale modifications when dimethyl carbonate is used as a co-solvent. The presence of an anion in the solvation shell of Li+ modifies the intermolecular structure comprising four carbonate molecules in dilute solutions, allowing only two carbonate molecules to coordinate to Li+. The bidentate complexation of Li+ with the anion’s electron donor atoms, however, maintains the local tetrahedral structure on interatomic length scales. The neutralization of the solvation shell of Li+ due to contact ion pair formation and the consequent implications on the underlying mechanisms provide a rational explanation for the ionic conductivity drop of electrolyte solutions at high salt concentrations.
中文翻译:

溶剂和盐对有机碳酸盐中锂离子溶剂化和接触离子对形成的影响:量子化学观点
已经采用量子化学计算来研究碳酸亚乙酯/碳酸亚丙酯和碳酸亚丙酯/碳酸二甲酯混合电解质中锂阳离子的溶剂化。抗衡阴离子的存在对Li +溶剂化的影响还研究了在纯碳酸亚丙酯和碳酸二甲酯中的含量。计算结果表明,在混合溶剂中,不同优选结构之间的跃迁具有较小的自由能变化。这意味着可以在混合物中进行不同的局部布置之间的过渡。碳酸二甲酯的添加导致溶剂化簇的偶极矩显着增加,表明当将碳酸二甲酯用作助溶剂时,重要的分子规模改性。Li +的溶剂化壳中阴离子的存在会改变分子溶液的分子结构,该分子结构在稀溶液中包含四个碳酸酯分子,仅两个碳酸酯分子能与Li +配位。Li +的双齿络合物然而,带有阴离子的电子给体原子的原子在原子间长度尺度上保持了局部四面体结构。由于接触离子对的形成,Li +的溶剂化壳被中和及其对潜在机理的影响,为高盐浓度下电解质溶液的离子电导率下降提供了合理的解释。
更新日期:2018-11-07
中文翻译:

溶剂和盐对有机碳酸盐中锂离子溶剂化和接触离子对形成的影响:量子化学观点
已经采用量子化学计算来研究碳酸亚乙酯/碳酸亚丙酯和碳酸亚丙酯/碳酸二甲酯混合电解质中锂阳离子的溶剂化。抗衡阴离子的存在对Li +溶剂化的影响还研究了在纯碳酸亚丙酯和碳酸二甲酯中的含量。计算结果表明,在混合溶剂中,不同优选结构之间的跃迁具有较小的自由能变化。这意味着可以在混合物中进行不同的局部布置之间的过渡。碳酸二甲酯的添加导致溶剂化簇的偶极矩显着增加,表明当将碳酸二甲酯用作助溶剂时,重要的分子规模改性。Li +的溶剂化壳中阴离子的存在会改变分子溶液的分子结构,该分子结构在稀溶液中包含四个碳酸酯分子,仅两个碳酸酯分子能与Li +配位。Li +的双齿络合物然而,带有阴离子的电子给体原子的原子在原子间长度尺度上保持了局部四面体结构。由于接触离子对的形成,Li +的溶剂化壳被中和及其对潜在机理的影响,为高盐浓度下电解质溶液的离子电导率下降提供了合理的解释。















































 京公网安备 11010802027423号
京公网安备 11010802027423号