当前位置:
X-MOL 学术
›
Chem. Eur. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
1,2,5‐Triphenylpyrrole Derivatives with Dual Intense Photoluminescence in Both Solution and the Solid State: Solvatochromism and Polymorphic Luminescence Properties
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2018-12-13 , DOI: 10.1002/chem.201804074
Yuanyuan Li 1 , Yunxiang Lei 2 , Lichao Dong 2 , Longlong Zhang 1 , Junge Zhi 1 , Jianbing Shi 2 , Bin Tong 2 , Yuping Dong 2
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2018-12-13 , DOI: 10.1002/chem.201804074
Yuanyuan Li 1 , Yunxiang Lei 2 , Lichao Dong 2 , Longlong Zhang 1 , Junge Zhi 1 , Jianbing Shi 2 , Bin Tong 2 , Yuping Dong 2
Affiliation
|
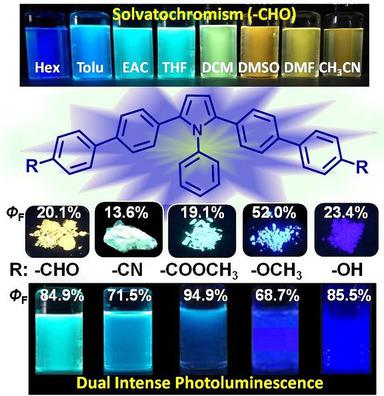
Five organic luminophores, 1,2,5‐triphenylpyrrole (TPP) derivatives 3 a–e bearing electron‐withdrawing or electron‐donating groups, have been synthesized by Pd‐catalyzed Suzuki coupling of 1‐phenyl‐2,5‐di(4′‐bromophenyl)pyrrole and para‐substituted phenylboronic acid derivatives. They possess good thermal stabilities with high decomposition temperatures above 310 °C. Investigation of the photophysical properties of the luminogens 3 a–e indicated that they exhibited dual intense photoluminescence in both solution and the solid state due to their twisted conformations, and their fluorescence quantum yields (ΦF) were determined as 68.7–94.9 % in THF solution and 19.1–52.0 % in solid powder form. Compounds 3 a–c bearing electron‐accepting groups exhibited remarkable solvatochromism with large Stokes shifts, attributable to their D‐π‐A structure and intramolecular charge‐transfer effect. In particular, 3 a, bearing aldehyde groups, displayed an obvious red‐shift of the emission band from 445 to 564 nm with increasing solvent polarity. However, no obvious solvatochromic behavior was observed for compounds 3 d,e bearing electron‐donating groups. The luminophore 3 a exhibited polymorphic luminescence properties and crystallization‐induced emission enhancement.
中文翻译:

溶液和固态均具有双重强光致发光的1,2,5-三苯基吡咯衍生物:溶剂致变色和多态发光性质
通过Pd催化的1-苯基-2,5-di(4)的Suzuki偶联反应合成了5个带有吸电子基团或供电子基团的1,2,5-三苯基吡咯(TPP)衍生物3 a – e有机发光体。' -溴苯基)吡咯和对位取代的苯基硼酸衍生物。它们具有良好的热稳定性,并具有高于310°C的高分解温度。所述luminogens的光物理性质的研究3 - ë由于它们的构象扭曲,和它们的荧光量子产率(表明它们表现出在溶液和固体状态的双光致发光强Φ ˚F)在THF溶液中的含量为68.7–94.9%,在固体粉末形式中的含量为19.1–52.0%。带有电子接受基团的化合物3 a – c表现出显着的溶剂致变色现象,具有大的斯托克斯位移,这归因于它们的D-π-A结构和分子内电荷转移效应。特别是,带有醛基的3 a随着溶剂极性的增加,发射带从445 nm明显迁移到564 nm。然而,观察到的化合物没有明显的溶剂化变色行为3 d,ê轴承供电子基团。发光体3a表现出多态发光特性和结晶诱导的发射增强。
更新日期:2018-12-13
中文翻译:

溶液和固态均具有双重强光致发光的1,2,5-三苯基吡咯衍生物:溶剂致变色和多态发光性质
通过Pd催化的1-苯基-2,5-di(4)的Suzuki偶联反应合成了5个带有吸电子基团或供电子基团的1,2,5-三苯基吡咯(TPP)衍生物3 a – e有机发光体。' -溴苯基)吡咯和对位取代的苯基硼酸衍生物。它们具有良好的热稳定性,并具有高于310°C的高分解温度。所述luminogens的光物理性质的研究3 - ë由于它们的构象扭曲,和它们的荧光量子产率(表明它们表现出在溶液和固体状态的双光致发光强Φ ˚F)在THF溶液中的含量为68.7–94.9%,在固体粉末形式中的含量为19.1–52.0%。带有电子接受基团的化合物3 a – c表现出显着的溶剂致变色现象,具有大的斯托克斯位移,这归因于它们的D-π-A结构和分子内电荷转移效应。特别是,带有醛基的3 a随着溶剂极性的增加,发射带从445 nm明显迁移到564 nm。然而,观察到的化合物没有明显的溶剂化变色行为3 d,ê轴承供电子基团。发光体3a表现出多态发光特性和结晶诱导的发射增强。































 京公网安备 11010802027423号
京公网安备 11010802027423号