当前位置:
X-MOL 学术
›
Nat. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mesopontine cholinergic inputs to midbrain dopamine neurons drive stress-induced depressive-like behaviors.
Nature Communications ( IF 14.7 ) Pub Date : 2018-10-25 , DOI: 10.1038/s41467-018-06809-7 Sebastian P Fernandez 1, 2 , Loïc Broussot 1, 2 , Fabio Marti 3, 4 , Thomas Contesse 1, 2 , Xavier Mouska 1, 2 , Mariano Soiza-Reilly 3, 5 , Hélène Marie 1, 2 , Philippe Faure 3, 4 , Jacques Barik 1, 2
Nature Communications ( IF 14.7 ) Pub Date : 2018-10-25 , DOI: 10.1038/s41467-018-06809-7 Sebastian P Fernandez 1, 2 , Loïc Broussot 1, 2 , Fabio Marti 3, 4 , Thomas Contesse 1, 2 , Xavier Mouska 1, 2 , Mariano Soiza-Reilly 3, 5 , Hélène Marie 1, 2 , Philippe Faure 3, 4 , Jacques Barik 1, 2
Affiliation
|
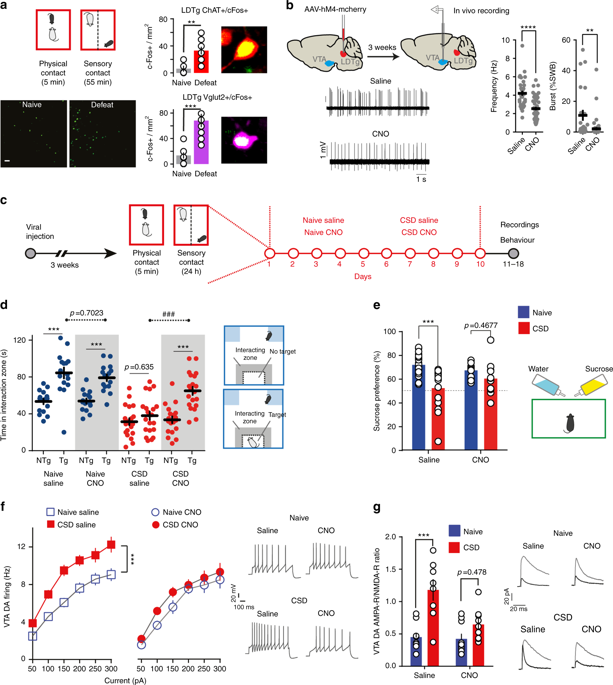
Stressful life events are primary environmental factors that markedly contribute to depression by triggering brain cellular maladaptations. Dysregulation of ventral tegmental area (VTA) dopamine neurons has been causally linked to the appearance of social withdrawal and anhedonia, two classical manifestations of depression. However, the relevant inputs that shape these dopamine signals remain largely unknown. We demonstrate that chronic social defeat (CSD) stress, a preclinical paradigm of depression, causes marked hyperactivity of laterodorsal tegmentum (LDTg) excitatory neurons that project to the VTA. Selective chemogenetic-mediated inhibition of cholinergic LDTg neurons prevent CSD-induced VTA DA neurons dysregulation and depressive-like behaviors. Pro-depressant outcomes are replicated by pairing activation of LDTg cholinergic terminals in the VTA with a moderate stress. Prevention of CSD outcomes are recapitulated by blocking corticotropin-releasing factor receptor 1 within the LDTg. These data uncover a neuro-circuitry of depressive-like disorders and demonstrate that stress, via a neuroendocrine signal, profoundly dysregulates the LDTg.
中文翻译:

中脑多巴胺神经元的中脑桥胆碱能输入驱动压力诱发的抑郁样行为。
生活压力事件是主要的环境因素,通过引发脑细胞适应不良,显着导致抑郁。腹侧被盖区(VTA)多巴胺神经元的失调与社交退缩和快感缺乏(抑郁症的两种典型表现)的出现存在因果关系。然而,塑造这些多巴胺信号的相关输入仍然很大程度上未知。我们证明,慢性社交失败(CSD)压力(抑郁症的一种临床前范例)会导致投射到 VTA 的后背被盖(LDTg)兴奋性神经元明显过度活跃。选择性化学遗传学介导的胆碱能 LDTg 神经元抑制可防止 CSD 诱导的 VTA DA 神经元失调和抑郁样行为。通过将 VTA 中的 LDTg 胆碱能末端激活与中等压力配对来复制促抑郁结果。通过阻断 LDTg 内的促肾上腺皮质激素释放因子受体 1 来预防 CSD 后果。这些数据揭示了抑郁样疾病的神经回路,并证明压力通过神经内分泌信号使 LDTg 严重失调。
更新日期:2018-10-25
中文翻译:

中脑多巴胺神经元的中脑桥胆碱能输入驱动压力诱发的抑郁样行为。
生活压力事件是主要的环境因素,通过引发脑细胞适应不良,显着导致抑郁。腹侧被盖区(VTA)多巴胺神经元的失调与社交退缩和快感缺乏(抑郁症的两种典型表现)的出现存在因果关系。然而,塑造这些多巴胺信号的相关输入仍然很大程度上未知。我们证明,慢性社交失败(CSD)压力(抑郁症的一种临床前范例)会导致投射到 VTA 的后背被盖(LDTg)兴奋性神经元明显过度活跃。选择性化学遗传学介导的胆碱能 LDTg 神经元抑制可防止 CSD 诱导的 VTA DA 神经元失调和抑郁样行为。通过将 VTA 中的 LDTg 胆碱能末端激活与中等压力配对来复制促抑郁结果。通过阻断 LDTg 内的促肾上腺皮质激素释放因子受体 1 来预防 CSD 后果。这些数据揭示了抑郁样疾病的神经回路,并证明压力通过神经内分泌信号使 LDTg 严重失调。































 京公网安备 11010802027423号
京公网安备 11010802027423号