当前位置:
X-MOL 学术
›
Nat. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
ALDH1A1 provides a source of meiosis-inducing retinoic acid in mouse fetal ovaries.
Nature Communications ( IF 14.7 ) Pub Date : 2016-Feb-19 , DOI: 10.1038/ncomms10845 Josephine Bowles , Chun-Wei Feng , Kim Miles , Jessica Ineson , Cassy Spiller , Peter Koopman
Nature Communications ( IF 14.7 ) Pub Date : 2016-Feb-19 , DOI: 10.1038/ncomms10845 Josephine Bowles , Chun-Wei Feng , Kim Miles , Jessica Ineson , Cassy Spiller , Peter Koopman
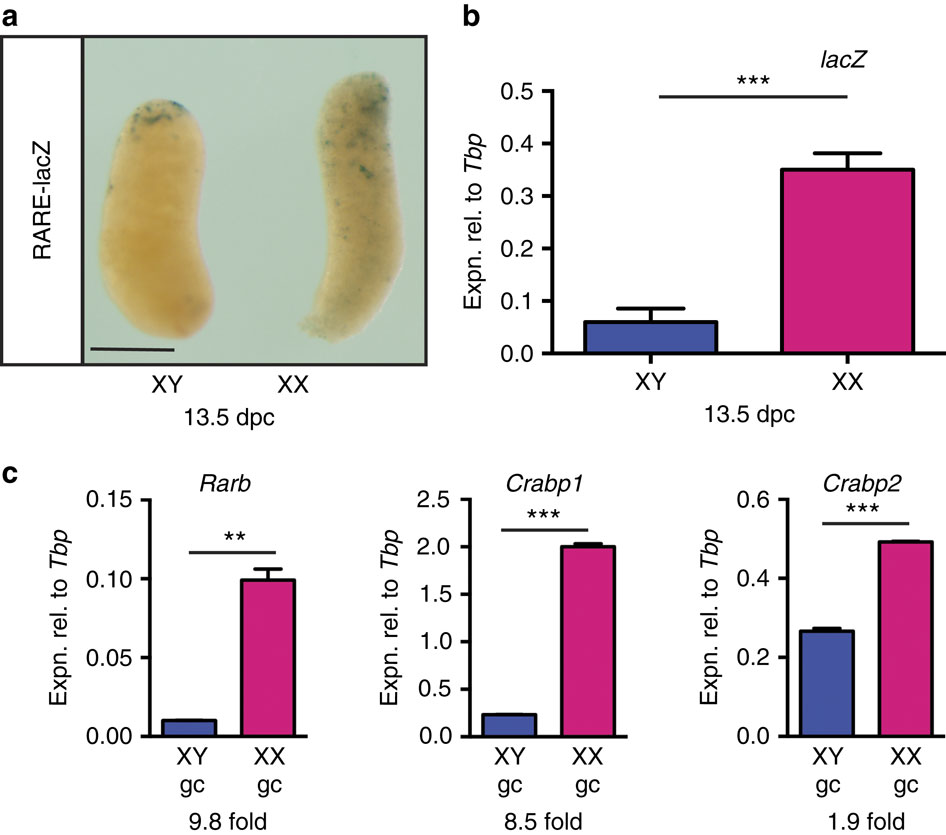
|
Substantial evidence exists that during fetal ovarian development in mammals, retinoic acid (RA) induces germ cells to express the pre-meiotic marker Stra8 and enter meiosis, and that these effects are prevented in the fetal testis by the RA-degrading P450 enzyme CYP26B1. Nonetheless, the role of RA has been disputed principally because germ cells in embryos lacking two major RA-synthesizing enzymes, ALDH1A2 and ALDH1A3, remain able to enter meiosis. Here we show that a third RA-synthesizing enzyme, ALDH1A1, is expressed in fetal ovaries, providing a likely source of RA in the absence of ALDH1A2 and ALDH1A3. In ovaries lacking ALDH1A1, the onset of germ cell meiosis is delayed. Our data resolve the conundrum posed by conflicting published data sets and reconfirm the model that meiosis is triggered by endogenous RA in the developing ovary.
中文翻译:

ALDH1A1提供了小鼠胎儿卵巢中诱导减数分裂的视黄酸来源。
大量证据表明,在哺乳动物胎儿卵巢发育过程中,视黄酸(RA)诱导生殖细胞表达减数分裂前标志物Stra8并进入减数分裂,并且通过降解RA的P450酶CYP26B1阻止了胎儿睾丸中的这些作用。尽管如此,RA的作用一直存在争议,主要是因为缺少两种主要RA合成酶ALDH1A2和ALDH1A3的胚胎中的生殖细胞仍然能够进入减数分裂。在这里,我们显示了第三个RA合成酶ALDH1A1在胎儿卵巢中表达,在没有ALDH1A2和ALDH1A3的情况下提供了RA的可能来源。在缺少ALDH1A1的卵巢中,生殖细胞减数分裂的发作被延迟。我们的数据解决了相互矛盾的已发布数据集所带来的难题,并再次证实了模型的减数分裂是由发育中的卵巢内源性RA触发的。
更新日期:2016-02-26
中文翻译:

ALDH1A1提供了小鼠胎儿卵巢中诱导减数分裂的视黄酸来源。
大量证据表明,在哺乳动物胎儿卵巢发育过程中,视黄酸(RA)诱导生殖细胞表达减数分裂前标志物Stra8并进入减数分裂,并且通过降解RA的P450酶CYP26B1阻止了胎儿睾丸中的这些作用。尽管如此,RA的作用一直存在争议,主要是因为缺少两种主要RA合成酶ALDH1A2和ALDH1A3的胚胎中的生殖细胞仍然能够进入减数分裂。在这里,我们显示了第三个RA合成酶ALDH1A1在胎儿卵巢中表达,在没有ALDH1A2和ALDH1A3的情况下提供了RA的可能来源。在缺少ALDH1A1的卵巢中,生殖细胞减数分裂的发作被延迟。我们的数据解决了相互矛盾的已发布数据集所带来的难题,并再次证实了模型的减数分裂是由发育中的卵巢内源性RA触发的。






























 京公网安备 11010802027423号
京公网安备 11010802027423号