当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Trigonal-Bipyramidal Coordination in First Ammoniates of ZnF2: ZnF2(NH3)3 and ZnF2(NH3)2
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2016-02-19 00:00:00 , DOI: 10.1021/acs.inorgchem.5b02837
Theresia M. M. Richter 1 , Sylvain LeTonquesse 1 , Nicolas S. A. Alt 2 , Eberhard Schlücker 2 , Rainer Niewa 1
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2016-02-19 00:00:00 , DOI: 10.1021/acs.inorgchem.5b02837
Theresia M. M. Richter 1 , Sylvain LeTonquesse 1 , Nicolas S. A. Alt 2 , Eberhard Schlücker 2 , Rainer Niewa 1
Affiliation
|
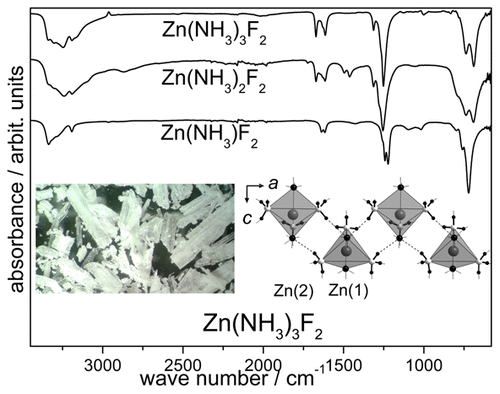
Single crystals of ZnF2(NH3)3 and ZnF2(NH3)2 were obtained under ammonothermal conditions (250 °C, 196 MPa and 500 °C, 136 MPa). Upon thermal decomposition of both ZnF2(NH3)3 and ZnF2(NH3)2, a microcrystalline powder of ZnF2(NH3) was obtained. ZnF2(NH3)3 and ZnF2(NH3)2 represent probable intermediates in a conceivable ammonothermal synthesis of the semiconductor Zn3N2 and manifest a rare trigonal-bipyramidal coordination of F– and NH3 ligands around Zn2+ according to single-crystal X-ray diffraction. Thermal analysis of all three compounds showed not only ZnF2(NH3) but also ZnF2(NH3)2 to be decomposition intermediates of ZnF2(NH3)3 prior to the formation of ZnF2. All three compounds demonstrate hydrogen bonds, as indicated by the intensities and half-widths of the bands in the vibrational spectra and by short N–H···F distances in the crystal structures of ZnF2(NH3)3 and ZnF2(NH3)2. With ZnF2(NH3)3, ZnF2(NH3)2, and ZnF2(NH3), we present the first ammoniates of ZnF2.
中文翻译:

ZnF 2:ZnF 2(NH 3)3和ZnF 2(NH 3)2的第一铵盐中的三角-双锥体配位
在氨热条件下(250℃,196MPa和500℃,136MPa )获得ZnF 2(NH 3)3和ZnF 2(NH 3)2的单晶。对双方的ZnF的热分解2(NH 3)3和的ZnF 2(NH 3)2,的ZnF的微晶粉末2(NH 3),得到。ZnF 2(NH 3)3和ZnF 2(NH 3)2表示可能的中间体,在半导体的Zn可想到氨热合成3 Ñ 2和清单f的罕见三角-双锥协调-和NH 3点周围的配体的Zn 2+根据单晶X射线衍射。对这三种化合物的热分析表明,在形成ZnF 2之前,不仅ZnF 2(NH 3)还是ZnF 2(NH 3)2都是ZnF 2(NH 3)3的分解中间体。。这三种化合物均表现出氢键,如振动谱带的强度和半峰宽度以及ZnF 2(NH 3)3和ZnF 2( NH 3)2。对于ZnF 2(NH 3)3,ZnF 2(NH 3)2和ZnF 2(NH 3),我们提出了ZnF 2的第一批氨化物。
更新日期:2016-02-19
中文翻译:

ZnF 2:ZnF 2(NH 3)3和ZnF 2(NH 3)2的第一铵盐中的三角-双锥体配位
在氨热条件下(250℃,196MPa和500℃,136MPa )获得ZnF 2(NH 3)3和ZnF 2(NH 3)2的单晶。对双方的ZnF的热分解2(NH 3)3和的ZnF 2(NH 3)2,的ZnF的微晶粉末2(NH 3),得到。ZnF 2(NH 3)3和ZnF 2(NH 3)2表示可能的中间体,在半导体的Zn可想到氨热合成3 Ñ 2和清单f的罕见三角-双锥协调-和NH 3点周围的配体的Zn 2+根据单晶X射线衍射。对这三种化合物的热分析表明,在形成ZnF 2之前,不仅ZnF 2(NH 3)还是ZnF 2(NH 3)2都是ZnF 2(NH 3)3的分解中间体。。这三种化合物均表现出氢键,如振动谱带的强度和半峰宽度以及ZnF 2(NH 3)3和ZnF 2( NH 3)2。对于ZnF 2(NH 3)3,ZnF 2(NH 3)2和ZnF 2(NH 3),我们提出了ZnF 2的第一批氨化物。






































 京公网安备 11010802027423号
京公网安备 11010802027423号