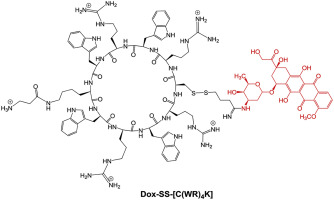
药物外排引起的心肌毒性和耐药性是基于多柔比星 (Dox) 的化疗的主要局限性。Dox 结构修饰可用于开发具有改进生物学特性的缀合物,例如抗增殖活性和更高的细胞保留。因此,Dox 硫醇缀合物、Dox 硫醇 (Dox-SH)、硫醇反应性 Dox-SS-吡啶(SS = 二硫化物)和 Dox-SS-细胞穿透环肽 Dox-SS-[C(WR) 4 K],被合成。Dox 与 Traut 试剂反应生成 Dox-SH。通过与二硫代二吡啶反应活化硫醇基团,得到相应的Dox-SS-吡啶(Dox-SS-Pyr)。含有半胱氨酸残基的环状细胞穿透肽 [C(WR) 4K] 是使用 Fmoc 固相策略制备的。Dox-SS-Py 与 [C(WR) 4 K]中半胱氨酸的游离巯基反应,生成 Dox-SS-[C(WR) 4 K] 作为 Dox 环肽缀合物。在人胚胎肾 (HEK-293)、人卵巢癌 (SKOV-3)、人纤维肉瘤 (HT-1080) 和人白血病 (CCRF-CEM) 细胞中检查了这些化合物的细胞毒性。与 Dox 相比,在 HEK-293、HT-1080 和 CCRF-CEM 细胞中培养 24 小时和 72 小时后,发现 Dox-SH 和 Dox-SS-吡啶具有显着更高或相当的细胞毒性,大概是因为更高的活性和保留这些细胞中的化合物。此外,Dox-SS-[C(WR) 4K] 在培养 72 小时后与 Dox 相比,在 HEK-293、HT-1080 和 SKOV-3 细胞中显示出显着更高的细胞毒活性。Dox-SS-Pyr在 HT-1080 和 HEK-293 细胞中表现出比 Dox-SS-[C(WR) 4 K]更高的细胞吸收,如流式细胞术所示。荧光显微镜显示 Dox-SS-Pyr、Dox-SH 和 Dox-SS-[C(WR) 4 K] 位于细胞核中,如四种细胞系所示,HT-1080、SKOV-3、MDA-MB- 468 和 MCF-7。值得注意的是,与相同浓度的 Dox 相比,Dox-SS-[C(WR) 4 K] 在小鼠成肌细胞中的毒性明显较低。进一步的机理研究表明,暴露于 Dox-SS-[C(WR) 4 的成肌细胞中细胞内活性氧 (ROS) 的水平当与 FeCl 2共同处理时,与 Dox 相比,K] 降低。这些数据表明 Dox-SH、Dox-SS-Pyr 和 Dox-SS-[C(WR) 4 K] 有可能作为 Dox 替代品和抗癌剂进行进一步研究。
 "点击查看英文标题和摘要"
"点击查看英文标题和摘要"
Synthesis and antiproliferative activities of doxorubicin thiol conjugates and doxorubicin-SS-cyclic peptide
Myocardial toxicity and drug resistance caused by drug efflux are major limitations of doxorubicin (Dox)-based chemotherapy. Dox structure modification could be used to develop conjugates with an improved biological profile, such as antiproliferative activity and higher cellular retention. Thus, Dox thiol conjugates, Dox thiol (Dox-SH), thiol-reactive Dox-SS-pyridine (SS = disulfide), and a Dox-SS-cell-penetrating cyclic peptide, Dox-SS-[C(WR)4K], were synthesized. Dox was reacted with Traut's reagent to generate Dox-SH. The thiol group was activated by the reaction with dithiodipyridine to afford the corresponding Dox-SS-Pyridine (Dox-SS-Pyr). A cyclic cell-penetrating peptide containing a cysteine residue [C(WR)4K] was prepared using Fmoc solid-phase strategy. Dox-SS-Py was reacted with the free sulfhydryl of cysteine in [C(WR)4K] to generate Dox-SS-[C(WR)4K] as a Dox-cyclic peptide conjugate. Cytotoxicity of the compounds was examined in human embryonic kidney (HEK-293), human ovarian cancer (SKOV-3), human fibrosarcoma (HT-1080), and human leukemia (CCRF-CEM) cells. Dox-SH and Dox-SS-pyridine were found to have significantly higher or comparable cytotoxicity when compared to Dox in HEK-293, HT-1080, and CCRF-CEM cells after 24 h and 72 incubation, presumably because of higher activity and retention of the compounds in these cells. Furthermore, Dox-SS-[C(WR)4K] showed significantly higher cytotoxic activity in HEK-293, HT-1080, and SKOV-3 cells when compared with Dox after 72 h incubation. Dox-SS-Pyr exhibited higher cellular uptake than Dox-SS-[C(WR)4K] in HT-1080 and HEK-293 cells as shown by flow cytometry. Fluorescence microscopy exhibited that Dox-SS-Pyr, Dox-SH, and Dox-SS-[C(WR)4K] localized in the nucleus as shown in four cell lines, HT-1080, SKOV-3, MDA-MB-468, and MCF-7. Of note, Dox-SS-[C(WR)4K] was significantly less toxic in mouse myoblast cells compared to Dox at the same concentration. Further mechanistic study demonstrated that the level of intracellular reactive oxygen species (ROS) in myoblast cells exposed to Dox-SS-[C(WR)4K] was reduced in comparison of Dox when co-treated with FeCl2. These data indicate that Dox-SH, Dox-SS-Pyr, and Dox-SS-[C(WR)4K] have the potential to be further examined as Dox alternatives and anticancer agents.
































 京公网安备 11010802027423号
京公网安备 11010802027423号