The EMBO Journal ( IF 9.4 ) Pub Date : 2018-11-15 , DOI: 10.15252/embj.2018100241 Andrés Norambuena 1 , Horst Wallrabe 1 , Rui Cao 2 , Dora Bigler Wang 1 , Antonia Silva 1 , Zdenek Svindrych 1, 3 , Ammasi Periasamy 1, 3 , Song Hu 2 , Rudolph E Tanzi 4 , Doo Yeon Kim 4 , George S Bloom 1, 5, 6
|
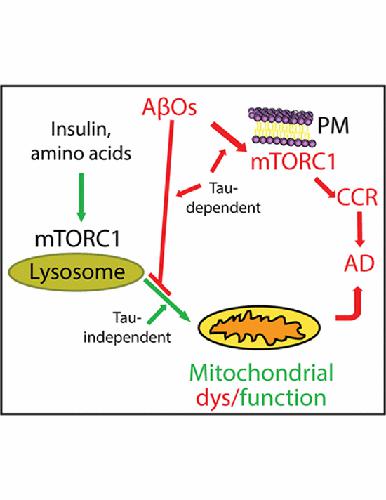
The mechanisms of mitochondrial dysfunction in Alzheimer's disease are incompletely understood. Using two‐photon fluorescence lifetime microscopy of the coenzymes, NADH and NADPH, and tracking brain oxygen metabolism with multi‐parametric photoacoustic microscopy, we show that activation of lysosomal mechanistic target of rapamycin complex 1 (mTORC1) by insulin or amino acids stimulates mitochondrial activity and regulates mitochondrial DNA synthesis in neurons. Amyloid‐β oligomers, which are precursors of amyloid plaques in Alzheimer's disease brain and stimulate mTORC1 protein kinase activity at the plasma membrane but not at lysosomes, block this Nutrient‐induced Mitochondrial Activity (NiMA) by a mechanism dependent on tau, which forms neurofibrillary tangles in Alzheimer's disease brain. NiMA was also disrupted in fibroblasts derived from two patients with tuberous sclerosis complex, a genetic disorder that causes dysregulation of lysosomal mTORC1. Thus, lysosomal mTORC1 couples nutrient availability to mitochondrial activity and links mitochondrial dysfunction to Alzheimer's disease by a mechanism dependent on the soluble building blocks of the poorly soluble plaques and tangles.
See also: JC Polanco & J Götz (November 2018)
中文翻译:

一种被淀粉样蛋白 β 寡聚体破坏的新型溶酶体到线粒体信号通路
阿尔茨海默病中线粒体功能障碍的机制尚不完全清楚。使用辅酶、NADH 和 NADPH 的双光子荧光寿命显微镜,并使用多参数光声显微镜跟踪脑氧代谢,我们表明胰岛素或氨基酸激活雷帕霉素复合物 1 (mTORC1) 的溶酶体机制靶标刺激线粒体活性并调节线粒体 DNA神经元中的合成。β 淀粉样蛋白寡聚体是阿尔茨海默病大脑中淀粉样斑块的前体,可刺激质膜但不刺激溶酶体的 mTORC1 蛋白激酶活性,通过依赖于 tau 的机制阻断这种营养诱导的线粒体活性 (NiMA),tau 在阿尔茨海默病大脑中形成神经原纤维缠结。NiMA 在源自两名结节性硬化症患者的成纤维细胞中也被破坏,结节性硬化症复合物是一种导致溶酶体 mTORC1 失调的遗传疾病。因此,溶酶体 mTORC1 将营养可用性与线粒体活性相结合,并通过依赖于难溶性斑块和缠结的可溶性组成部分的机制将线粒体功能障碍与阿尔茨海默病联系起来。
参见:JC Polanco & J Götz(2018年11月)


















































 京公网安备 11010802027423号
京公网安备 11010802027423号