当前位置:
X-MOL 学术
›
Nano Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Direction Matters: Monovalent Streptavidin/Biotin Complex under Load.
Nano Letters ( IF 9.6 ) Pub Date : 2018-10-22 00:00:00 , DOI: 10.1021/acs.nanolett.8b04045 Steffen M Sedlak 1 , Leonard C Schendel 1 , Marcelo C R Melo , Diana A Pippig 1 , Zaida Luthey-Schulten , Hermann E Gaub 1 , Rafael C Bernardi
Nano Letters ( IF 9.6 ) Pub Date : 2018-10-22 00:00:00 , DOI: 10.1021/acs.nanolett.8b04045 Steffen M Sedlak 1 , Leonard C Schendel 1 , Marcelo C R Melo , Diana A Pippig 1 , Zaida Luthey-Schulten , Hermann E Gaub 1 , Rafael C Bernardi
Affiliation
|
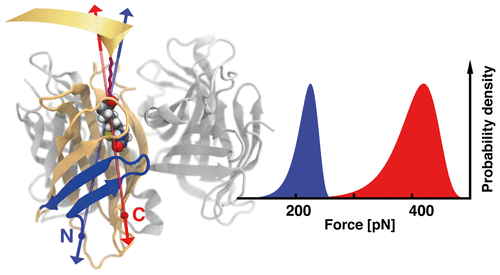
Novel site-specific attachment strategies combined with improvements of computational resources enable new insights into the mechanics of the monovalent biotin/streptavidin complex under load and forced us to rethink the diversity of rupture forces reported in the literature. We discovered that the mechanical stability of this complex depends strongly on the geometry in which force is applied. By atomic force microscopy-based single molecule force spectroscopy we found unbinding of biotin to occur beyond 400 pN at force loading rates of 10 nN/s when monovalent streptavidin was tethered at its C-terminus. This value is about twice as high than that for N-terminal attachment. Steered molecular dynamics simulations provided a detailed picture of the mechanics of the unbinding process in the corresponding force loading geometries. Using machine learning techniques, we connected findings from hundreds of simulations to the experimental results, identifying different force propagation pathways. Interestingly, we observed that depending on force loading geometry, partial unfolding of N-terminal region of monovalent streptavidin occurs before biotin is released from the binding pocket.
中文翻译:

方向很重要:负载下的单价链霉亲和素/生物素复合物。
新颖的位点特异性附着策略与计算资源的改进相结合,使人们能够对负载下单价生物素/链霉亲和素复合物的力学有了新的认识,并迫使我们重新思考文献中报道的断裂力的多样性。我们发现,这种复合体的机械稳定性很大程度上取决于施加力的几何形状。通过基于原子力显微镜的单分子力光谱,我们发现当单价链霉亲和素被束缚在其 C 末端时,在 10 nN/s 的力加载速率下,生物素会在超过 400 pN 时发生解结合。该值大约是 N 端连接值的两倍。引导分子动力学模拟提供了相应力加载几何结构中解绑过程力学的详细图片。使用机器学习技术,我们将数百次模拟的结果与实验结果联系起来,识别出不同的力传播路径。有趣的是,我们观察到,根据力加载几何形状,单价链霉亲和素 N 端区域的部分展开发生在生物素从结合袋释放之前。
更新日期:2018-10-22
中文翻译:

方向很重要:负载下的单价链霉亲和素/生物素复合物。
新颖的位点特异性附着策略与计算资源的改进相结合,使人们能够对负载下单价生物素/链霉亲和素复合物的力学有了新的认识,并迫使我们重新思考文献中报道的断裂力的多样性。我们发现,这种复合体的机械稳定性很大程度上取决于施加力的几何形状。通过基于原子力显微镜的单分子力光谱,我们发现当单价链霉亲和素被束缚在其 C 末端时,在 10 nN/s 的力加载速率下,生物素会在超过 400 pN 时发生解结合。该值大约是 N 端连接值的两倍。引导分子动力学模拟提供了相应力加载几何结构中解绑过程力学的详细图片。使用机器学习技术,我们将数百次模拟的结果与实验结果联系起来,识别出不同的力传播路径。有趣的是,我们观察到,根据力加载几何形状,单价链霉亲和素 N 端区域的部分展开发生在生物素从结合袋释放之前。

































 京公网安备 11010802027423号
京公网安备 11010802027423号