Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Investigation of coal particle gasification processes with application leading to underground coal gasification
Fuel ( IF 6.7 ) Pub Date : 2019-02-01 , DOI: 10.1016/j.fuel.2018.10.058 Tata Sutardi , Manosh C. Paul , Nader Karimi
Fuel ( IF 6.7 ) Pub Date : 2019-02-01 , DOI: 10.1016/j.fuel.2018.10.058 Tata Sutardi , Manosh C. Paul , Nader Karimi
|
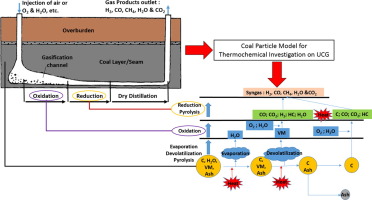
Abstract A coal particle model is developed to investigate the thermochemical processes of gasification for underground coal applications. The chemical reactions are defined with an Eddy Break up (EBU) model for controlling the reaction mechanisms and the study is particularly focused on identification of the important kinetic parameters, which control the consumption rate of coal mass. As an initial validation, the coal particle oxidation based on the experimental results is used for comparison. The gasification reactions are subsequently applied for the thermochemical process investigation, and the results show that the best agreement of coal oxidation is achieved by the pre-exponent factor (A) of 0.002 and 85500, for the reactions, R2 (C + O2 = CO2) and R3 (C + 0.5O2 = CO), respectively. The kinetic parameters for the gasification process of coal particle leading to the syngas production are also optimised. The results show that the production of H2 and CO is controlled significantly by the level of oxygen concentration in the char reactions. However, their chemical rates are strongly dependent upon the reaction zones. For example, CO is produced in both the oxidation and reduction reaction zones, while H2 production is dominated in the reduction zone. Spatio-temporal distributions of the gas species along with the coal particle temperature provide additional information for further development of UCG modelling. Ultimately, the model gives a good guideline with the associated thermochemical processes that can help developing advanced coal gasification technology and lead to improved syngas quality.
中文翻译:

煤颗粒气化工艺的研究与应用导致煤炭地下气化
摘要 开发了煤颗粒模型来研究地下煤应用的气化热化学过程。化学反应是用涡流分解 (EBU) 模型定义的,用于控制反应机制,研究特别侧重于识别控制煤质量消耗率的重要动力学参数。作为初步验证,基于实验结果的煤颗粒氧化用于比较。随后将气化反应应用于热化学过程研究,结果表明煤氧化的最佳一致性是由 0.002 和 85500 的前指数因子 (A) 实现的,对于反应,R2 (C + O2 = CO2 ) 和 R3 (C + 0.5O2 = CO),分别。还优化了导致合成气生产的煤颗粒气化过程的动力学参数。结果表明,H2 和CO 的产生受到炭反应中氧浓度水平的显着控制。然而,它们的化学速率强烈依赖于反应区。例如,在氧化和还原反应区都产生 CO,而在还原区主要产生 H2。气体种类的时空分布以及煤颗粒温度为 UCG 建模的进一步发展提供了额外的信息。最终,该模型为相关的热化学过程提供了良好的指导,有助于开发先进的煤气化技术并提高合成气质量。
更新日期:2019-02-01
中文翻译:

煤颗粒气化工艺的研究与应用导致煤炭地下气化
摘要 开发了煤颗粒模型来研究地下煤应用的气化热化学过程。化学反应是用涡流分解 (EBU) 模型定义的,用于控制反应机制,研究特别侧重于识别控制煤质量消耗率的重要动力学参数。作为初步验证,基于实验结果的煤颗粒氧化用于比较。随后将气化反应应用于热化学过程研究,结果表明煤氧化的最佳一致性是由 0.002 和 85500 的前指数因子 (A) 实现的,对于反应,R2 (C + O2 = CO2 ) 和 R3 (C + 0.5O2 = CO),分别。还优化了导致合成气生产的煤颗粒气化过程的动力学参数。结果表明,H2 和CO 的产生受到炭反应中氧浓度水平的显着控制。然而,它们的化学速率强烈依赖于反应区。例如,在氧化和还原反应区都产生 CO,而在还原区主要产生 H2。气体种类的时空分布以及煤颗粒温度为 UCG 建模的进一步发展提供了额外的信息。最终,该模型为相关的热化学过程提供了良好的指导,有助于开发先进的煤气化技术并提高合成气质量。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号