Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2018-10-22 , DOI: 10.1016/j.cej.2018.10.159 Wonmi Lee , Agnesia Permatasari , Byeong Wan Kwon , Yongchai Kwon
|
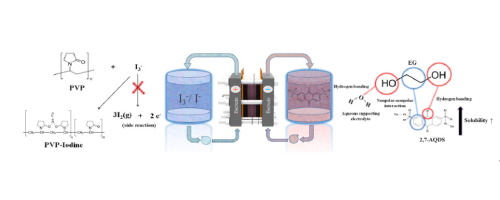
An aqueous organic redox flow battery (AORFB) using anthraquinone-2,7-disulfonic acid disodium salt (2,7-AQDS) and potassium iodide (KI) as the negative and positive active species is suggested. The active species are dissolved into an aqueous potassium chloride (KCl) solution, while ethylene glycol (EG) and polyvinylpyrrolidone (PVP) are added to improve the solubility of 2,7-AQDS and prevent the side reactions of KI. The aqueous solubility of redox couple improved by the utilization of additive plays a role in increasing both capacity and the performance of AORFB, while as kinetic parameters to affect the capacity, electron transfer rate constant (ks) and diffusion coefficient (D) are measured. As a result, when EG is used, the solubility of 2,7-AQDS increases from 0.3 to 0.8 M in KCl solution and PVP acts as a barrier preventing the production of iodine gas that is a product generated by the side reaction of KI. The increase in solubility by the use of EG is because it has two hydroxyl groups and they form hydrogen bonding with the oxygen of sulfonyl or carbonyl group within 2,7-AQDS, while the alkyl group of its backbone that is in non-polar nature interacts with the phenyl group within 2,7-AQDS. In addition, EG increases the redox activity of KI because this significantly increases the nucleophilic ability of the iodide anion. Regarding the effect of PVP, when PVP is added to the solution containing iodine gas, povidone-iodine complex is formed and this complex impedes the side reaction of KI and maintains iodate that is a reactant for redox reaction as it is in the system. With that, when the AORFB using the 2,7-AQDS and KI including EG and PVP is run for ten cycle, it shows excellent performances like the discharge capacity of 0.7 AhL−1 and the coulombic and energy efficiencies of 98% and 82%.
中文翻译:

蒽醌-2,7-二磺酸二钠盐和碘化钾氧化还原对对水性有机氧化还原液流电池性能的评估
建议使用蒽醌-2,7-二磺酸二钠盐(2,7-AQDS)和碘化钾(KI)作为负极和正极活性物质的有机氧化还原液流电池(AORFB)。将活性物质溶解在氯化钾(KCl)水溶液中,同时添加乙二醇(EG)和聚乙烯吡咯烷酮(PVP)以提高2,7-AQDS的溶解度并防止KI的副反应。通过使用添加剂改善的氧化还原对的水溶解度在增加容量和AORFB的性能方面都起着作用,而作为影响容量的动力学参数,电子传递速率常数(k s)和扩散系数(D)的测量。结果,当使用EG时,2,7-AQDS在KCl溶液中的溶解度从0.3M增加到0.8M,并且PVP充当阻挡层,防止产生碘气,碘气是由KI的副反应产生的产物。通过使用EG来增加溶解度是因为它具有两个羟基,并且它们与2,7-AQDS中的磺酰基或羰基的氧形成氢键,而其骨架的烷基是非极性的与2,7-AQDS中的苯基相互作用。另外,EG增加了KI的氧化还原活性,因为这显着增加了碘化物阴离子的亲核能力。关于PVP的效果,将PVP添加到含碘气体的溶液中时,形成聚维酮-碘络合物,该络合物阻碍了KI的副反应,并保持了碘酸盐,碘酸盐是氧化还原反应的反应物,与系统中的状态一样。这样,当使用包含EG和PVP的2,7-AQDS和KI的AORFB运行十个周期时,它表现出出色的性能,例如0.7 AhL的放电容量-1,库仑和能源效率分别为98%和82%。







































 京公网安备 11010802027423号
京公网安备 11010802027423号