Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2018-10-21 , DOI: 10.1016/j.bmc.2018.10.018
Ping Zhou , Gang Chen , Minqi Gao , Jiaquan Wu
|
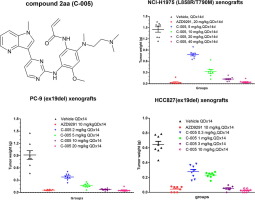
Osimertinib has been approved as a first-line treatment for non-small-cell lung cancer (NSCLC) patients whose tumor carries EGFR activation and / or resistant mutations. To mitigate Osimertinib’s toxicity caused by AZ5104, the N-demethylation metabolite of Osimertinib, we designed and synthesized a series of Osimertinib analogs with different headpieces. In vitro and in vivo analysis rendered a potential clinical candidate C-005 which had pyrrolo-pyridine headpiece. Biochemically, C-005 and its main human hepatocyte metabolite showed over 30 fold selectivity of L858R/T790M mutant EGFR over WT EGFR. Such selectivity profile was retained at cellular level. In general, C-005 is 2-14 fold more selective than Osimertinib in a panel of WT EGFR cancer cell lines. Furthermore, C-005 demonstrated robust antitumor efficacy and good tolerability in NCI-H1975, PC-9 and HCC827 xenograft mouse models, making it a potential candidate for human test in clinical.
中文翻译:

奥西替尼类似物(C-005)作为针对NSCLC的强力EGFR抑制剂的设计,合成和评估
Osimertinib已被批准作为其肿瘤带有EGFR激活和/或耐药突变的非小细胞肺癌(NSCLC)患者的一线治疗药物。为了减轻由奥西替尼的N-去甲基代谢产物AZ5104引起的奥西替尼的毒性,我们设计并合成了一系列具有不同头戴装置的奥西替尼类似物。体外和体内分析提供了具有吡咯并吡啶头帽的潜在临床候选C-005。生化方面,C-005及其主要的人类肝细胞代谢产物显示L858R / T790M突变型EGFR的选择性是野生型EGFR的30倍以上。这样的选择性分布在细胞水平上得以保留。通常,在一组WT EGFR癌细胞系中,C-005的选择性比奥西替尼高2-14倍。此外,C-005在NCI-H1975中表现出强大的抗肿瘤功效和良好的耐受性,































 京公网安备 11010802027423号
京公网安备 11010802027423号