当前位置:
X-MOL 学术
›
J. Agric. Food Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
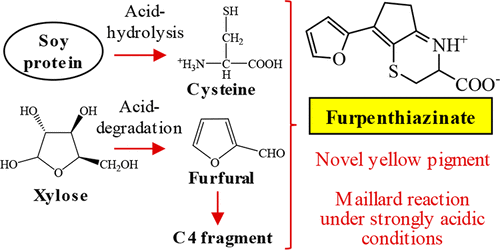
Novel Maillard Pigment, Furpenthiazinate, Having Furan and Cyclopentathiazine Rings Formed by Acid Hydrolysis of Protein in the Presence of Xylose or by Reaction between Cysteine and Furfural under Strongly Acidic Conditions
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2018-10-19 00:00:00 , DOI: 10.1021/acs.jafc.8b05302 Kyoko Noda , Ruriko Masuzaki , Yuka Terauchi , Shinji Yamada , Masatsune Murata
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2018-10-19 00:00:00 , DOI: 10.1021/acs.jafc.8b05302 Kyoko Noda , Ruriko Masuzaki , Yuka Terauchi , Shinji Yamada , Masatsune Murata
|
A novel Maillard pigment having partial structures of furan and cyclopentathiazine, named furpenthiazinate, was isolated and identified. Although this pigment was found in an acid hydrolysate of a Maillard reaction solution between soy protein and xylose, the same pigment was also formed by the Maillard reaction under strongly acidic conditions between soy protein and xylose and cysteine and furfural. The structure of its reduced form by NaBH4 was determined by MS, NMR, and X-ray analysis and identified as 7-(2-furanyl)-2,3,4,4a,5,6-hexahydrocyclopenta[b][1,4]thiazin-4-ium-3-carboxylate, indicating that the chemical structure of furpenthiazinate is 7-(2-furanyl)-2,3,5,6-tetrahydrocyclopenta[b][1,4]thiazine-3-carboxylic acid. Furpenthiazinate showed an absorption maximum at 400 nm and strong yellow color under acidic and neutral conditions. The color contribution of furpenthiazinate was estimated to be more than 60% in a reaction solution prepared from cysteine and furfural.
中文翻译:

具有木糖存在下酸水解蛋白质或强酸性条件下半胱氨酸与糠醛反应形成的新型美拉德颜料呋喃戊二酸酯,具有呋喃和环戊噻嗪环
分离并鉴定了具有呋喃和环戊二嗪部分结构的新型美拉德颜料,称为呋喃噻嗪酸盐。尽管在大豆蛋白和木糖之间的美拉德反应溶液的酸水解物中发现了这种颜料,但是在大豆蛋白和木糖以及半胱氨酸和糠醛之间的强酸性条件下,通过美拉德反应也形成了相同的颜料。通过MS,NMR和X射线分析确定了NaBH 4还原形式的结构,并将其鉴定为7-(2-呋喃基)-2,3,4,4a,5,6-六氢环戊[[ b ] [1] ,4] thiazin-4-ium-3-羧酸盐,表明呋喃噻嗪酸盐的化学结构为7-(2-呋喃基)-2,3,5,6-四氢环戊[ b] [1,4]噻嗪-3-羧酸。呋喃噻嗪酸盐在400 nm处显示最大吸收,在酸性和中性条件下呈强烈的黄色。在由半胱氨酸和糠醛制备的反应溶液中,呋喃噻嗪酸盐的颜色贡献估计超过60%。
更新日期:2018-10-19
中文翻译:

具有木糖存在下酸水解蛋白质或强酸性条件下半胱氨酸与糠醛反应形成的新型美拉德颜料呋喃戊二酸酯,具有呋喃和环戊噻嗪环
分离并鉴定了具有呋喃和环戊二嗪部分结构的新型美拉德颜料,称为呋喃噻嗪酸盐。尽管在大豆蛋白和木糖之间的美拉德反应溶液的酸水解物中发现了这种颜料,但是在大豆蛋白和木糖以及半胱氨酸和糠醛之间的强酸性条件下,通过美拉德反应也形成了相同的颜料。通过MS,NMR和X射线分析确定了NaBH 4还原形式的结构,并将其鉴定为7-(2-呋喃基)-2,3,4,4a,5,6-六氢环戊[[ b ] [1] ,4] thiazin-4-ium-3-羧酸盐,表明呋喃噻嗪酸盐的化学结构为7-(2-呋喃基)-2,3,5,6-四氢环戊[ b] [1,4]噻嗪-3-羧酸。呋喃噻嗪酸盐在400 nm处显示最大吸收,在酸性和中性条件下呈强烈的黄色。在由半胱氨酸和糠醛制备的反应溶液中,呋喃噻嗪酸盐的颜色贡献估计超过60%。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号