当前位置:
X-MOL 学术
›
Nat. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Self-powered H2 production with bifunctional hydrazine as sole consumable.
Nature Communications ( IF 14.7 ) Pub Date : 2018-10-19 , DOI: 10.1038/s41467-018-06815-9 Xijun Liu , Jia He , Shunzheng Zhao , Yunpeng Liu , Zhe Zhao , Jun Luo , Guangzhi Hu , Xiaoming Sun , Yi Ding
Nature Communications ( IF 14.7 ) Pub Date : 2018-10-19 , DOI: 10.1038/s41467-018-06815-9 Xijun Liu , Jia He , Shunzheng Zhao , Yunpeng Liu , Zhe Zhao , Jun Luo , Guangzhi Hu , Xiaoming Sun , Yi Ding
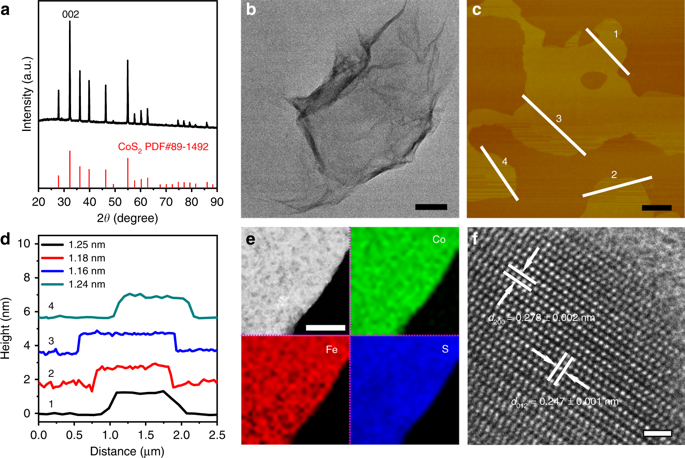
|
Splitting hydrazine into H2 and N2 by electro-catalyzing hydrogen evolution and hydrazine oxidation reactions is promising for replacing fossil energy with H2. However, current hydrazine splitting is achieved using external powers to drive the two reactions, which is inapplicable to outdoor use. Here, Fe-doped CoS2 nanosheets are developed as a bifunctional electrocatalyst for the two reactions, by which direct hydrazine fuel cells and overall-hydrazine-splitting units are realized and integrated to form a self-powered H2 production system. Without external powers, this system employs hydrazine bifunctionally as the fuel of direct hydrazine fuel cell and the splitting target, namely a sole consumable, and exhibits an H2 evolution rate of 9.95 mmol h-1, a 98% Faradaic efficiency and a 20-h stability, all comparable to the best reported for self-powered water splitting. These performances are due to that Fe doping decreases the free-energy changes of H adsorption and adsorbed NH2NH2 dehydrogenation on CoS2.
中文翻译:

使用双功能肼作为自备动力的自备氢气生产。
通过电催化析氢和肼氧化反应将肼分解为H 2和N 2有望用H 2代替化石能。然而,当前的肼分解是使用外部动力来驱动两个反应而实现的,不适用于室外。在这里,Fe掺杂的CoS 2纳米片被开发为用于两个反应的双功能电催化剂,通过该功能,可实现直接肼燃料电池和整体肼分解单元的整合,形成自供电的H 2。生产系统。该系统无需外部电源,就可以双功能使用肼作为直接肼燃料电池和分裂目标(即唯一的消耗品)的燃料,并具有9.95 mmol h -1的H 2释放速率,98%的法拉第效率和20 - H h稳定性,可与自供电水分解的最佳报告相媲美。这些性能是由于Fe掺杂降低了CoS 2上的H吸附和NH 2 NH 2脱氢吸附的自由能变化。
更新日期:2018-10-19
中文翻译:

使用双功能肼作为自备动力的自备氢气生产。
通过电催化析氢和肼氧化反应将肼分解为H 2和N 2有望用H 2代替化石能。然而,当前的肼分解是使用外部动力来驱动两个反应而实现的,不适用于室外。在这里,Fe掺杂的CoS 2纳米片被开发为用于两个反应的双功能电催化剂,通过该功能,可实现直接肼燃料电池和整体肼分解单元的整合,形成自供电的H 2。生产系统。该系统无需外部电源,就可以双功能使用肼作为直接肼燃料电池和分裂目标(即唯一的消耗品)的燃料,并具有9.95 mmol h -1的H 2释放速率,98%的法拉第效率和20 - H h稳定性,可与自供电水分解的最佳报告相媲美。这些性能是由于Fe掺杂降低了CoS 2上的H吸附和NH 2 NH 2脱氢吸附的自由能变化。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号