当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
NBO Orbital Interaction Analysis for the Ambiphilic Metal–Ligand Activation/Concerted Metalation Deprotonation (AMLA/CMD) Mechanism Involved in the Cyclopalladation Reaction of N,N-Dimethylbenzylamine with Palladium Acetate
Organometallics ( IF 2.5 ) Pub Date : 2018-10-19 , DOI: 10.1021/acs.organomet.8b00303 M. Arif Sajjad 1 , John A. Harrison 1 , Alastair J. Nielson 1 , Peter Schwerdtfeger 2
Organometallics ( IF 2.5 ) Pub Date : 2018-10-19 , DOI: 10.1021/acs.organomet.8b00303 M. Arif Sajjad 1 , John A. Harrison 1 , Alastair J. Nielson 1 , Peter Schwerdtfeger 2
Affiliation
|
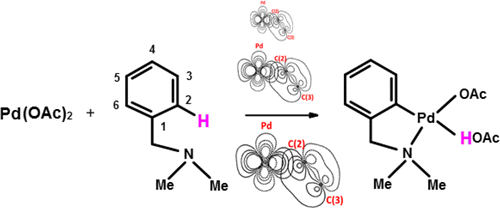
Natural bond orbital (NBO) analysis obtained from density functional theory (DFT) calculations on the intermediates and transition states in the ambiphilic metal–ligand activation/concerted metalation deprotonation (AMLA/CMD) mechanism for the cyclopalladation reaction of N,N-dimethylbenzylamine with palladium acetate shows the agostic, syndetic (π or σ-electron density from the ring that assists the agostic donation), and backbonding orbital overlaps involved. The analysis shows that these components are absent for the anagostic intermediate but progressively increase in going from the anagostic/agostic transition state to the agostic intermediate and then to the agostic/cyclopalladate transition state. For the all-important agostic/cyclopalladate transition state, agostic donation is very large [NBO E(2) total, 152.3 kcal mol–1], the syndetic π-donations total over half of this at 106.3 kcal mol–1 with the overlap forming in close proximity to the carbon where palladation occurs, and there is the emergence of σ-agostic donation (3.2 kcal mol–1). In comparison to the agostic intermediate, Pd to C–Hσ* back bonding increases marginally and C═O lone pair donation to this antibonding orbital increases significantly. The total back-donations have an E(2) value of 72.7 kcal mol–1, which is nearly half the magnitude of the agostic donation and provides a significant influence on the lengthening of the C–H bond.
中文翻译:

NBO轨道相互作用分析涉及N,N-二甲基苄胺与乙酸钯环化反应的两亲性金属-配体活化/一致的金属化去质子化(AMLA / CMD)机理
从密度泛函理论(DFT)计算得出的自然键轨道(NBO)分析中,两性金属,配体活化/固相金属化去质子化(AMLA / CMD)机制中的N和N的环化反应的中间体和过渡态-二甲基苄胺与乙酸钯表现出束缚,共形的(来自环的π或σ电子密度,有助于束缚捐献),并且涉及回键轨道重叠。分析表明,无味中间体不存在这些成分,但从无味/无味过渡态到含糊中间体,再到无味/环palladate过渡态,这些成分逐渐增加。对于最重要的偏北/环戊二烯过渡态,偏北捐赠非常大[NBO E(2)总计,152.3 kcal mol –1 ],辛迪加π-捐赠总计超过一半,为106.3 kcal mol –1随着重叠的形成,碳会非常靠近发生palpalation的地方,并且出现了σ-声波捐赠(3.2 kcal mol –1)。与原始中间体相比,Pd到C–Hσ *的反向键略有增加,并且向该反键轨道的C═O孤对捐赠显着增加。总回赠的E(2)值为72.7 kcal mol –1,几乎是无偿捐赠的一半,并且对C–H键的延长产生了重大影响。
更新日期:2018-10-19
中文翻译:

NBO轨道相互作用分析涉及N,N-二甲基苄胺与乙酸钯环化反应的两亲性金属-配体活化/一致的金属化去质子化(AMLA / CMD)机理
从密度泛函理论(DFT)计算得出的自然键轨道(NBO)分析中,两性金属,配体活化/固相金属化去质子化(AMLA / CMD)机制中的N和N的环化反应的中间体和过渡态-二甲基苄胺与乙酸钯表现出束缚,共形的(来自环的π或σ电子密度,有助于束缚捐献),并且涉及回键轨道重叠。分析表明,无味中间体不存在这些成分,但从无味/无味过渡态到含糊中间体,再到无味/环palladate过渡态,这些成分逐渐增加。对于最重要的偏北/环戊二烯过渡态,偏北捐赠非常大[NBO E(2)总计,152.3 kcal mol –1 ],辛迪加π-捐赠总计超过一半,为106.3 kcal mol –1随着重叠的形成,碳会非常靠近发生palpalation的地方,并且出现了σ-声波捐赠(3.2 kcal mol –1)。与原始中间体相比,Pd到C–Hσ *的反向键略有增加,并且向该反键轨道的C═O孤对捐赠显着增加。总回赠的E(2)值为72.7 kcal mol –1,几乎是无偿捐赠的一半,并且对C–H键的延长产生了重大影响。





























 京公网安备 11010802027423号
京公网安备 11010802027423号