当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Regioselective Metal-Free Aza-Heck Reactions of Terminal Alkenes Catalyzed by Phosphine Selenides
Organic Letters ( IF 4.9 ) Pub Date : 2018-10-19 00:00:00 , DOI: 10.1021/acs.orglett.8b03159 Tianyi Zheng 1 , John R. Tabor 1 , Zackary L. Stein 1 , Forrest E. Michael 1
Organic Letters ( IF 4.9 ) Pub Date : 2018-10-19 00:00:00 , DOI: 10.1021/acs.orglett.8b03159 Tianyi Zheng 1 , John R. Tabor 1 , Zackary L. Stein 1 , Forrest E. Michael 1
Affiliation
|
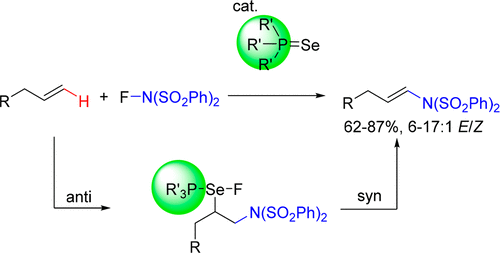
Phosphine selenides are introduced as an alternate class of selenium-based catalysts for the aza-Heck reaction of alkenes. Using these catalysts, a range of terminal alkenes react with NFBS to give oxidative amination products. Judicious choice of phosphine ligand gives greater regio- and stereoselectivity than with diphenyl diselenide, enabling the selective formation of E terminal enimides in high yields. Isotope-labeling experiments and measurements of kinetic isotope effects reveal that the reaction occurs stereospecifically via irreversible anti addition, followed by rate-determining syn elimination.
中文翻译:

膦膦硒化物催化末端烯烃的区域选择性无金属氮杂-Heck反应
硒化膦作为烯烃的氮杂-Heck反应的另一类硒基催化剂而引入。使用这些催化剂,一定范围的末端烯烃与NFBS反应生成氧化胺化产物。与二苯基二硒化物相比,明智地选择膦配体可提供更高的区域选择性和立体选择性,从而能够高收率地选择性形成E末端亚胺。同位素标记实验和动力学同位素效应的测量结果表明,该反应通过不可逆的抗加成反应立体定向地发生,然后通过速率决定性的syn消除。
更新日期:2018-10-19
中文翻译:

膦膦硒化物催化末端烯烃的区域选择性无金属氮杂-Heck反应
硒化膦作为烯烃的氮杂-Heck反应的另一类硒基催化剂而引入。使用这些催化剂,一定范围的末端烯烃与NFBS反应生成氧化胺化产物。与二苯基二硒化物相比,明智地选择膦配体可提供更高的区域选择性和立体选择性,从而能够高收率地选择性形成E末端亚胺。同位素标记实验和动力学同位素效应的测量结果表明,该反应通过不可逆的抗加成反应立体定向地发生,然后通过速率决定性的syn消除。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号