当前位置:
X-MOL 学术
›
Eur. J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
PdII‐Mediated Oxidative Amination for Access to a 9‐Azabicyclo[4.2.1]nonane Compound Library and Anatoxin‐a
European Journal of Organic Chemistry ( IF 2.5 ) Pub Date : 2018-10-18 , DOI: 10.1002/ejoc.201801209 Rafid S. Dawood 1, 2 , Suresh R. Chidipudi 1 , Daniel C. O'Connor 1 , William Lewis 1 , Daniel Hamza 3 , Christopher A. Pearce 3 , Geraint Jones 3 , Ross P. Wilkie 1 , Irene Georgiou 1 , Thomas E. Storr 1 , Jonathan C. Moore 1 , Robert A. Stockman 1
European Journal of Organic Chemistry ( IF 2.5 ) Pub Date : 2018-10-18 , DOI: 10.1002/ejoc.201801209 Rafid S. Dawood 1, 2 , Suresh R. Chidipudi 1 , Daniel C. O'Connor 1 , William Lewis 1 , Daniel Hamza 3 , Christopher A. Pearce 3 , Geraint Jones 3 , Ross P. Wilkie 1 , Irene Georgiou 1 , Thomas E. Storr 1 , Jonathan C. Moore 1 , Robert A. Stockman 1
Affiliation
|
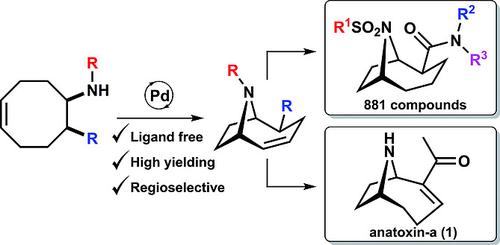
Biologically relevant 9 azabicyclo[4.2.1]nonanes can be synthesised through an intramolecular oxidative amination of aminocyclooct‐4‐enes. The reaction is generally high yielding, has good substrate scope and proceeds under “ligand‐free” catalytic conditions. The protocol was applied to the synthesis of anatoxin‐a and a series of chemical scaffolds, which were further derivatised to form an 881‐membered compound library.

中文翻译:

PdII介导的氧化胺可访问9-氮杂双环[4.2.1]壬烷化合物库和Anatoxin-a
与生物相关的9个氮杂双环[4.2.1]壬烷可以通过氨基环辛-4-烯的分子内氧化胺化反应合成。该反应通常收率高,具有良好的底物范围,可在“无配体”催化条件下进行。该方案适用于合成Anatoxin-a和一系列化学支架,并将其进一步衍生化以形成881元化合物库。

更新日期:2018-10-18

中文翻译:

PdII介导的氧化胺可访问9-氮杂双环[4.2.1]壬烷化合物库和Anatoxin-a
与生物相关的9个氮杂双环[4.2.1]壬烷可以通过氨基环辛-4-烯的分子内氧化胺化反应合成。该反应通常收率高,具有良好的底物范围,可在“无配体”催化条件下进行。该方案适用于合成Anatoxin-a和一系列化学支架,并将其进一步衍生化以形成881元化合物库。






















































 京公网安备 11010802027423号
京公网安备 11010802027423号